Chapter: Basic & Clinical Pharmacology : Antiprotozoal Drugs
Atovaquone - Malaria
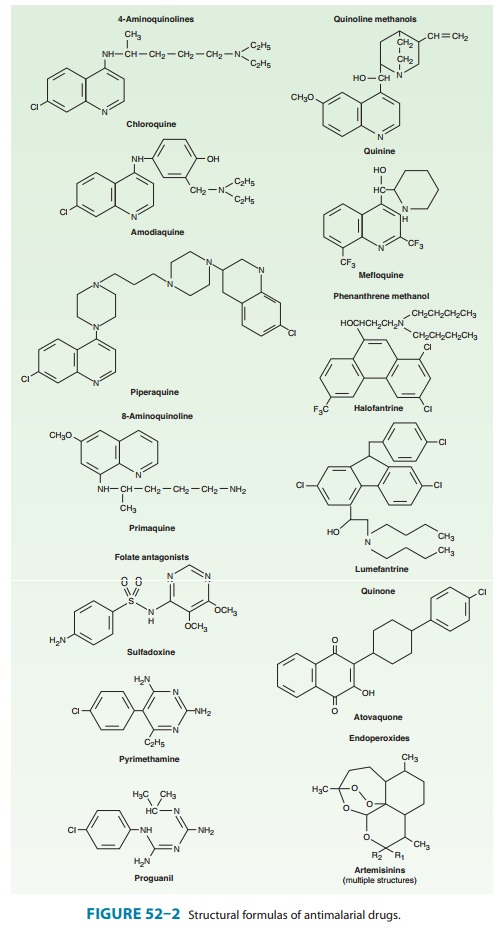
ATOVAQUONE
Atovaquone,
a hydroxynaphthoquinone (Figure 52–2), was initially developed as an
antimalarial agent, and as a component of Malarone
is recommended for treatment and prevention of malaria. Atovaquone has also
been approved by the FDA for the treatment of mild to moderate P jiroveci pneumonia.
The
drug is only administered orally. Its bioavailability is low and erratic, but absorption
is increased by fatty food. The drug is heavily protein-bound and has a
half-life of 2–3 days. Most of the drug is eliminated unchanged in the feces.
Atovaquone acts against plasmodia by disrupting mitochondrial electron
transport. It is active against tissue and erythrocytic schizonts, allowing
chemoprophylaxis to be discontinued only 1 week after the end of exposure
(compared with 4 weeks for mefloquine or doxycycline, which lack activity
against tissue schizonts).
Initial use of
atovaquone to treat malaria led to disappointing results, with frequent
failures, apparently due to the selection of resistant parasites during
therapy. In contrast, Malarone, a fixed combination of atovaquone (250 mg) and
proguanil (100 mg), is highly effective for both the treatment and
chemoprophylaxis of falciparum malaria, and it is now approved for both
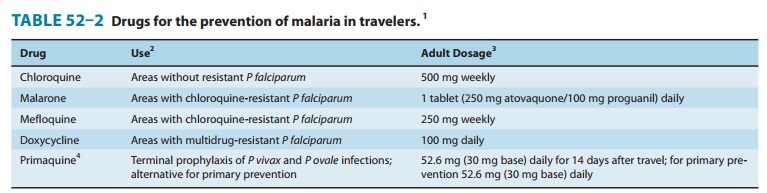
indications in the USA. For chemoprophylaxis, Malarone must be taken daily
(Table 52–2). It has an advantage over mefloquine and doxycy-cline in requiring
shorter periods of treatment before and after the period at risk for malaria
transmission, but it is more expensive than the other agents. It should be
taken with food.
Atovaquone is an
alternative therapy for P jiroveci
infection, although its efficacy is lower than that of
trimethoprim-sulfamethoxazole. Standard dosing is 750 mg taken with food twice
daily for 21 days. Adverse effects include fever, rash, nausea, vomiting,
diarrhea, headache, and insomnia. Serious adverse effects appear to be
mini-mal, although experience with the drug remains limited. Atovaquone has
also been effective in small numbers of immunocompromised patients with
toxoplasmosis unresponsive to other agents, although its role in this disease
is not yet defined.
Malarone
is generally well tolerated. Adverse effects include abdominal pain, nausea,
vomiting, diarrhea, headache, and rash, and these are more common with the
higher dosage required for treatment. Reversible elevations in liver enzymes
have been reported. The safety of atovaquone in pregnancy is unknown. Plasma
concentrations of atovaquone are decreased about 50% by co-administration of
tetracycline or rifampin.
Related Topics