Chapter: Basic & Clinical Pharmacology : Antiprotozoal Drugs
Mefloquine - Malaria
MEFLOQUINE
Mefloquine is
effective therapy for many chloroquine-resistant strains of P falciparum and against other species.
Although toxicity is a concern, mefloquine is one of the recommended
chemoprophylactic drugs for use in most malaria-endemic regions with
chloroquine-resistant strains.
Chemistry & Pharmacokinetics
Mefloquine
hydrochloride is a synthetic 4-quinoline methanol that is chemically related to
quinine. It can only be given orally because severe local irritation occurs
with parenteral use. It is well absorbed, and peak plasma concentrations are
reached in about 18 hours. Mefloquine is highly protein-bound, extensively
distrib-uted in tissues, and eliminated slowly, allowing a single-dose
treat-ment regimen. The terminal elimination half-life is about 20 days,
allowing weekly dosing for chemoprophylaxis. With weekly dos-ing, steady-state
drug levels are reached over a number of weeks; this interval can be shortened
to 4 days by beginning a course with three consecutive daily doses of 250 mg,
although this is not stan-dard practice. Mefloquine and acid metabolites of the
drug are slowly excreted, mainly in the feces. The drug can be detected in the
blood for months after the completion of therapy.
Antimalarial Action & Resistance
Mefloquine has strong
blood schizonticidal activity against P
falciparum and P vivax, but it is
not active against hepatic stagesor gametocytes. The mechanism of action of
mefloquine is unknown. Sporadic resistance to mefloquine has been reported from
many areas. At present, resistance appears to be uncommon except in regions of
Southeast Asia with high rates of multidrug resistance (especially border areas
of Thailand). Mefloquine resis-tance appears to be associated with resistance
to quinine and halofantrine but not with resistance to chloroquine.
Clinical Uses
A. Chemoprophylaxis
Mefloquine is
effective in prophylaxis against most strains of P falciparum and probably all other human malarial
species.Mefloquine is therefore among the drugs recommended by the CDC for
chemoprophylaxis in all malarious areas except for those with no chloroquine
resistance (where chloroquine is preferred) and some rural areas of Southeast
Asia with a high prevalence of mefloquine resistance. As with chloroquine,
eradication of P vivax and P ovale requires a course of primaquine.
B. Treatment
Mefloquine is
effective in treating most falciparum malaria. The drug is not appropriate for
treating individuals with severe or complicated malaria, since quinine,
quinidine, and artemisinins are more rapidly active, and since drug resistance
is less likely with those agents. The combination of artesunate plus mefloquine
showed excellent antimalarial efficacy in regions of Southeast Asia with some
resistance to mefloquine, and this regimen is now oneof the combination
therapies recommended by the WHO for the treatment of uncomplicated falciparum
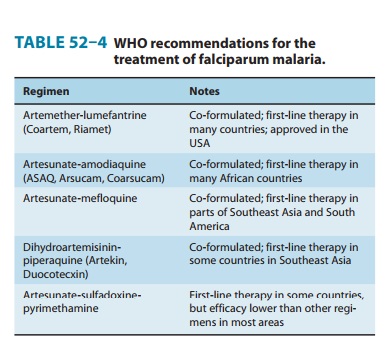
malaria (Table 52–4). Artesunate-mefloquine is the first-line therapy for uncomplicated
malaria in a number of countries in Asia and South America.
Adverse Effects
Weekly dosing with
mefloquine for chemoprophylaxis may cause nausea, vomiting, dizziness, sleep
and behavioral disturbances, epigastric pain, diarrhea, abdominal pain, headache,
rash, and diz-ziness. Neuropsychiatric toxicities have received a good deal of
publicity, but despite frequent anecdotal reports of seizures and psychosis, a
number of controlled studies have found the fre-quency of serious adverse
effects from mefloquine to be no higher than that with other common
antimalarial chemoprophylactic regimens. Leukocytosis, thrombocytopenia, and
aminotransferase elevations have been reported.
The latter adverse
effects are more common with the higher dosages required for treatment. These
effects may be lessened by administering the drug in two doses separated by 6–8
hours. The incidence of neuropsychiatric symptoms appears to be about ten times
more common than with chemoprophylactic dosing, with widely varying frequencies
of up to about 50% being reported. Serious neuropsychiatric toxicities
(depression, confusion, acute psychosis, or seizures) have been reported in
less than 1 in 1000 treatments, but some authorities believe that these
toxicities are actually more common. Mefloquine can also alter cardiac
conduc-tion, and arrhythmias and bradycardia have been reported.
Contraindications & Cautions
Mefloquine is
contraindicated in a patient with a history of epi-lepsy, psychiatric
disorders, arrhythmia, cardiac conduction defects, or sensitivity to related
drugs. It should not be co-administered with quinine, quinidine, or
halofantrine, and caution is required if quinine or quinidine is used to treat
malaria after mefloquine chemoprophylaxis. Theoretical risks of mefloquine must
be bal-anced with the risk of contracting falciparum malaria. The CDC no longer
advises against mefloquine use in patients receiving β-adrenoceptor antagonists. Mefloquine is also
now consideredsafe in young children. Available data suggest that mefloquine is
safe throughout pregnancy, although experience in the first trimes-ter is
limited. An older recommendation to avoid mefloquine use in those requiring
fine motor skills (eg, airline pilots) is controver-sial. Mefloquine
chemoprophylaxis should be discontinued if sig-nificant neuropsychiatric
symptoms develop.
Related Topics