Chapter: Basic & Clinical Pharmacology : Antiprotozoal Drugs
Inhibitors of Folate Synthesis - Malaria
INHIBITORS OF FOLATE SYNTHESIS
Inhibitors of enzymes
involved in folate metabolism are used, generally in combination regimens, in
the treatment and prevention of malaria.
Chemistry & Pharmacokinetics
Pyrimethamine is
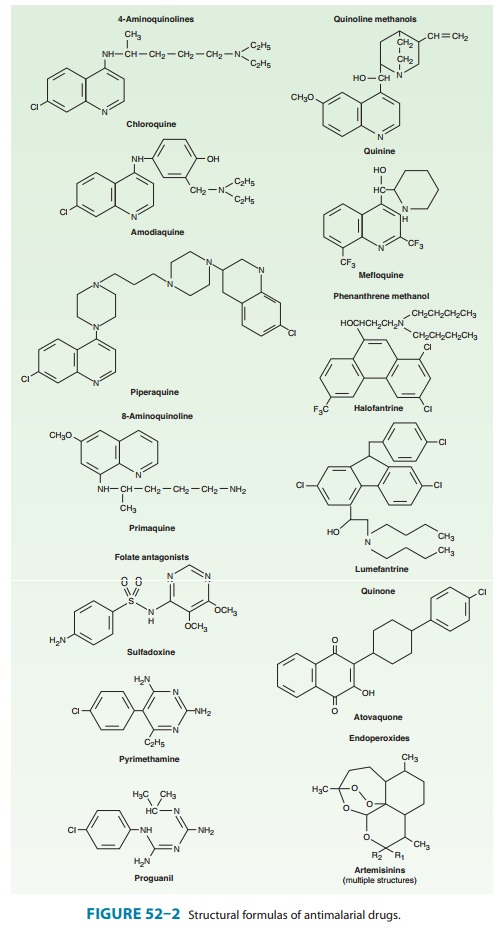
a 2,4-diaminopyrimidine related to trimetho-prim . Proguanil is a biguanide derivative (Figure 52–2). Both drugs are
slowly but adequately absorbed from the gastrointestinal tract. Pyrimethamine
reaches peak plasma lev-els 2–6 hours after an oral dose, is bound to plasma
proteins, and has an elimination half-life of about 3.5 days. Proguanil reaches
peak plasma levels about 5 hours after an oral dose and has an elimination
half-life of about 16 hours. Therefore, proguanil must be administered daily
for chemoprophylaxis, whereas pyrimethamine can be given once a week.
Pyrimethamine is extensively metabolized before excretion. Proguanil is a
prodrug; only its triazine metabolite, cycloguanil, is active. Fansidar, a fixed combination of the
sulfon-amide sulfadoxine (500 mg per
tablet) and pyrimethamine (25 mg per
tablet), is well absorbed. Its components display peak plasma levels within 2–8
hours and are excreted mainly by the kidneys. The average half-life of
sulfadoxine is about 170 hours.
Antimalarial Action & Resistance
Pyrimethamine
and proguanil act slowly against erythrocytic forms of susceptible strains of
all four human malaria species. Proguanil also has some activity against
hepatic forms. Neither drug is adequately gametocidal or effective against the
persistent liver stages of P vivax or
P ovale. Sulfonamides and sulfones
are weakly active against erythrocytic schizonts but not against liver stages
or gametocytes. They are not used alone as antimalarials but are effective in
combination with other agents.
The
mechanism of action of pyrimethamine and proguanil involves selective
inhibition of plasmodial dihydrofolate reductase, a key enzyme in the pathway
for synthesis of folate. Sulfonamides and sulfones inhibit another enzyme in
the folate pathway, dihy-dropteroate synthase. As described and shown in Figure
46–2, combinations of inhibitors of these two enzymes provide synergistic
activity.
Resistance to folate
antagonists and sulfonamides is common in many areas for P falciparum and less common for P vivax. Resistance is due primarily to mutations in dihydrofolate
reductase and dihydropteroate synthase, with increasing numbers of muta-tions
leading to increasing levels of resistance. At present, resis-tance seriously
limits the efficacy of sulfadoxine-pyrimethamine (Fansidar) for the treatment
of malaria in most areas, but in Africa most parasites exhibit an intermediate
level of resistance, such that antifolates may continue to offer some
preventive efficacy against malaria. Because different mutations may mediate
resistance to different agents, cross-resistance is not uniformly seen.
Clinical Uses
A. Chemoprophylaxis
Chemoprophylaxis
with single folate antagonists is no longer rec-ommended because of frequent
resistance, but a number of agents are used in combination regimens. The
combination of chloroquine (500 mg weekly) and proguanil (200 mg daily) was
previously widely used, but with increasing resistance to both agents it is no
longer recommended. Fansidar and Maloprim (the latter is a com-bination of
pyrimethamine and the sulfone dapsone) are both effective against sensitive
parasites with weekly dosing, but they are no longer recommended because of
resistance and toxicity. Considering protection of populations in endemic
regions, trimethoprim-sulfamethoxazole, an antifolate combination that is more
active against bacteria than malaria parasites, is increas-ingly used as a
daily prophylactic therapy for HIV-infected patients in developing countries.
Although it is administered primarily to prevent typical HIV opportunistic and
bacterial infections, this regimen offers partial preventive efficacy against
malaria in Africa.
B. Intermittent Preventive Therapy
A new strategy for
malaria control is intermittent preventive therapy, in which high-risk patients
receive intermittent treatment for malaria, regardless of their infection
status, typically with Fansidar, which benefits from simple dosing and
prolonged activ-ity. Considering the two highest risk groups for severe malaria
in Africa, this strategy is best validated in pregnant women and is
increasingly studied in young children. Typical schedules include single doses
of Fansidar during the second and third trimesters of pregnancy and monthly
doses whenever children present for scheduled immunizations or, in areas with
seasonal malaria, monthly doses during the transmission season. However,
optimal preventive dosing schedules have not been established.
C. Treatment of Chloroquine-Resistant Falciparum Malaria
Fansidar is commonly
used to treat uncomplicated falciparum malaria and until recently it was a
first-line therapy for this indica-tion in some tropical countries. Advantages
of Fansidar are ease of administration (a single oral dose) and low cost.
However, rates of resistance are increasing, and Fansidar is no longer a
recom-mended therapy. In particular, Fansidar should not be used for severe
malaria, since it is slower-acting than other available agents. Fansidar is
also not reliably effective in vivax malaria, and its use-fulness against P ovale and P malariae has not been adequately studied. A new
antifolate-sulfone combination, chlorproguanil-dapsone (Lapdap), was until
recently available in some African countries for the treatment of uncomplicated
falciparum malaria, and the combination of chlorproguanil-dapsone and
artesunate (Dacart) was under development. However, this project was
dis-continued in 2008 as a result of concerns about hematologic tox-icity in
those with G6PD deficiency, and chlorproguanil-dapsone will no longer be
marketed.
D. Toxoplasmosis
Pyrimethamine,
in combination with sulfadiazine, is first-line therapy in the treatment of
toxoplasmosis, including acute infec-tion, congenital infection, and disease in
immunocompromised patients. For immunocompromised patients, high-dose therapy
is required followed by chronic suppressive therapy. Folinic acid is included
to limit myelosuppression. Toxicity from the combina-tion is usually due primarily
to sulfadiazine. The replacement of sulfadiazine with clindamycin provides an
effective alternative regimen.
E. Pneumocystosis
P jiroveci is the cause of human pneumocystosis and is now recog-nized to
be a fungus, but this organism is discussed because it responds to
antiprotozoal drugs, not antifungals. (The related species P carinii is now recognized to be the cause of ani-mal infections.)
First-line therapy of pneumocystosis is trimetho-prim plus sulfamethoxazole.
Standard treatment includes high-dose intravenous or oral therapy (15 mg
trimethoprim and 75 mg sulfamethoxazole per day in three or four divided doses)
for 21 days. High-dose therapy entails signifi-cant toxicity, especially in
patients with AIDS. Important toxici-ties include nausea, vomiting, fever,
rash, leukopenia, hyponatremia, elevated hepatic enzymes, azotemia, anemia, and
thrombocytope-nia. Less common effects include severe skin reactions, mental
status changes, pancreatitis, and hypocalcemia. Trimethoprim-sulfamethoxazole
is also the standard chemoprophylactic drug for the prevention of P jiroveci
infection in immunocompromised individuals. Dosing is one double-strength
tablet daily or three times per week. The chemoprophylactic dosing schedule is
much better tolerated than high-dose therapy in immunocompromised patients, but
rash, fever, leukopenia, or hepatitis may necessitate changing to another drug.
Adverse Effects & Cautions
Most patients tolerate
pyrimethamine and proguanil well. Gastrointestinal symptoms, skin rashes, and
itching are rare. Mouth ulcers and alopecia have been described with proguanil.
Fansidar is no longer recommended for chemoprophylaxis because of uncommon but
severe cutaneous reactions, including erythema multiforme, Stevens-Johnson
syndrome, and toxic epidermal necrolysis. Severe reactions appear to be much
less common with single-dose or intermittent therapy, and use of the drug has
been justified by the risks associated with falciparum malaria.
Rare adverse effects
with a single dose of Fansidar are those associated with other sulfonamides,
including hematologic, gas-trointestinal, central nervous system, dermatologic,
and renal toxicity. Maloprim is no longer recommended for chemoprophy-laxis
because of unacceptably high rates of agranulocytosis. Folate antagonists
should be used cautiously in the presence of renal or hepatic dysfunction.
Although pyrimethamine is teratogenic in animals, Fansidar has been safely used
in pregnancy for therapy and as an intermittent chemoprophylactic regimen to
improve pregnancy outcomes. Proguanil is considered safe in pregnancy. Folate
supplements should be routinely administered during preg-nancy, but in women
receiving Fansidar preventive therapy, high-dose folate supplementation (eg, 5
mg daily) should probably be avoided because it may limit preventive efficacy.
The standard recommended dosage of 0.4–0.6 mg daily is less likely to affect
Fansidar’s protective efficacy.
Related Topics