Chapter: Clinical Anesthesiology: Regional Anesthesia & Pain Management: Chronic Pain Management
Physiology of Nociception
PHYSIOLOGY OF NOCICEPTION
1. Nociceptors
Nociceptors are characterized by a high threshold for activation and
encode the intensity of stimula-tion by increasing their discharge rates in a
graded fashion. Following repeated stimulation, they char-acteristically
display delayed adaptation, sensitiza-tion, and afterdischarges.
Noxious sensations can often be broken down
into two components: a fast, sharp, and well-localized sensation (“first pain”), which
is conducted with a short latency (0.1 s) by Aδ fibers (tested by pin-prick); and a slower onset, duller, and often
poorly localized sensation (“second pain”), which is con-ducted by C fibers. In
contrast to epicritic sensation, which may be transduced by specialized end
organs on the afferent neuron (eg, pacinian corpuscle for touch), protopathic
sensation is transduced mainly by free nerve endings.
Most nociceptors are free nerve endings that
sense heat and mechanical and chemical tissue damage. Types include (1)
mechanonociceptors, which respond to pinch and pinprick, (2) silent
nociceptors, which respond only in the presence of inflammation, and (3)
polymodal mechanoheat nociceptors. The last are most prevalent and respond to
excessive pressure, extremes of temperature (>42°C and <40°C), and noxious substances such as bradykinin, histamine, serotonin
(5-hydroxytrypta-mine or 5-HT), H+, K+, some prostaglandins, capsa-icin, and
possibly adenosine triphosphate. At least two nociceptor receptors (containing
ion channels in nerve endings) have been identified, TRPV1 and TRPV2. Both
respond to high temperatures. Cap-saicin stimulates the TRPV1 receptor.
Polymodal nociceptors are slow to adapt to strong pressure and display heat
sensitization.
Cutaneous Nociceptors
Nociceptors are present in both somatic and
visceral tissues. Primary afferent neurons reach tissues by traveling along
spinal somatic, sympathetic, or para-sympathetic nerves. Somatic nociceptors
include those in skin (cutaneous) and deep tissues (muscle, tendons, fascia,
and bone), whereas visceral nocicep-tors include those in internal organs. The
cornea and tooth pulp are unique in that they are almost exclu-sively
innervated by nociceptive Aδ and C fibers.
Deep Somatic Nociceptors
Deep somatic nociceptors are less sensitive to nox-ious stimuli than
cutaneous nociceptors but are easily sensitized by inflammation. The pain
arising from them is characteristically dull and poorly local-ized. Specific
nociceptors exist in muscles and joint capsules, and they respond to
mechanical, thermal, and chemical stimuli.
Visceral Nociceptors
Visceral organs are generally insensitive tissues that mostly contain
silent nociceptors. Some organs appear to have specif ci nociceptors, such as
the heart, lung, testis, and bile ducts. Most other organs, such as the
intestines, are innervated by polymodal nociceptors that respond to smooth
muscle spasm, ischemia, and inflammation. These receptors gen-erally do not
respond to the cutting, burning, or crushing that occurs during surgery. A few
organs, such as the brain, lack nociceptors altogether; how-ever, the brain’s
meningeal coverings do contain nociceptors.
Like somatic nociceptors, those in the viscera are the free nerve
endings of primary afferent neu-rons whose cell bodies lie in the dorsal horn.
These afferent nerve fibers, however, frequently travel with efferent
sympathetic nerve fibers to reach the viscera. Afferent activity from these
neurons enters the spinal cord between T1 and L2. Nociceptive C fibers from the
esophagus, larynx, and trachea travel with the vagus nerve to enter the nucleus
sol-itarius in the brainstem. Afferent pain fibers from the bladder, prostate,
rectum, cervix and urethra, and genitalia are transmitted into the spinal cord
via parasympathetic nerves at the level of the S2–S4 nerve roots. Though
relatively few compared with somatic pain fibers, fibers from primary visceral
afferent neurons enter the cord and synapse more diffusely with single fibers,
often synapsing with multiple dermatomal levels and often crossing to the
contralateral dorsal horn.
2. Chemical Mediators of Pain
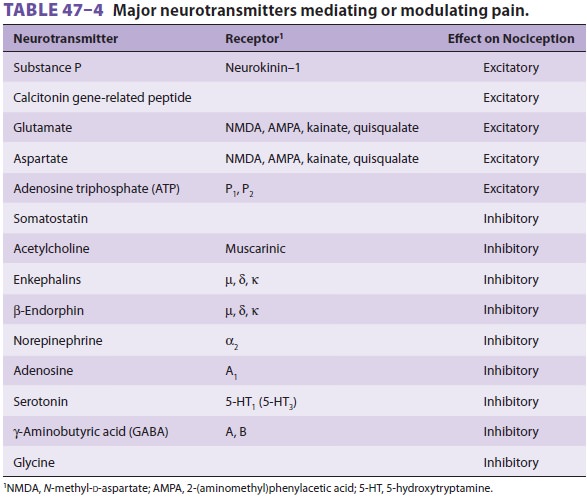
Several neuropeptides and excitatory amino
acids function as neurotransmitters for afferent neurons subserving pain (Table
47–4). Many, if not most, of these neurons
contain more than one neurotrans-mitter, which are simultaneously released. The
most important of these peptides are substance P and calcitonin gene-related
peptide (CGRP). Glutamate is the most important excitatory amino acid.Substance
P is an 11 amino acid peptide that is synthesized and released by first-order
neurons both peripherally and in the dorsal horn. Also found in other parts of
the nervous system and the intestines
it facilitates transmission in pain pathways via neu-rokinin-1 receptor
activation. In the periphery, sub-stance P neurons send collaterals that are
closely associated with blood vessels, sweat glands, hair follicles, and mast
cells in the dermis. Substance P sensitizes nociceptors, degranulates histamine
from mast cells and 5-HT from platelets, and is a potent vasodilator and
chemoattractant for leukocytes. Substance P–releasing neurons also innervate
the viscera and send collateral fibers to paravertebral sympathetic ganglia;
intense stimulation of viscera, therefore, can cause direct postganglionic
sympa-thetic discharge.
Both opioid and α2-adrenergic
receptors have been described on or near the terminals of unmy-elinated
peripheral nerves. Although their physi-ological role is not clear, the latter
may explain the observed analgesia of peripherally applied opioids,
particularly in the presence of inflammation.
3. Modulation of Pain
Modulation of pain occurs peripherally at the
nociceptor, in the spinal cord, and in supraspinal structures. This modulation
can either inhibit (suppress) or facilitate (intensify) pain.
Peripheral Modulation of Pain
Nociceptors and their neurons display sensitization following repeated
stimulation. Sensitization may be manifested as an enhanced response to noxious
stimulation or a newly acquired responsiveness to a wider range of stimuli,
including nonnoxious stimuli.
A. Primary Hyperalgesia
Sensitization of nociceptors results in a decrease in threshold, an
increase in the frequency response to the same stimulus intensity, a decrease
in response latency, and spontaneous firing even after cessation
of the stimulus (afterdischarges). Such sensitization commonly occurs
with injury and following applica-tion of heat. Primary hyperalgesia is
mediated by the release of noxious substances from damaged tissues. Histamine
is released from mast cells, basophils, and platelets, whereas serotonin is
released from mast cells and platelets. Bradykinin is released from tissues
following activation of factor XII. Bradyki-nin activates free nerve endings
via specific B1 and B2 receptors.
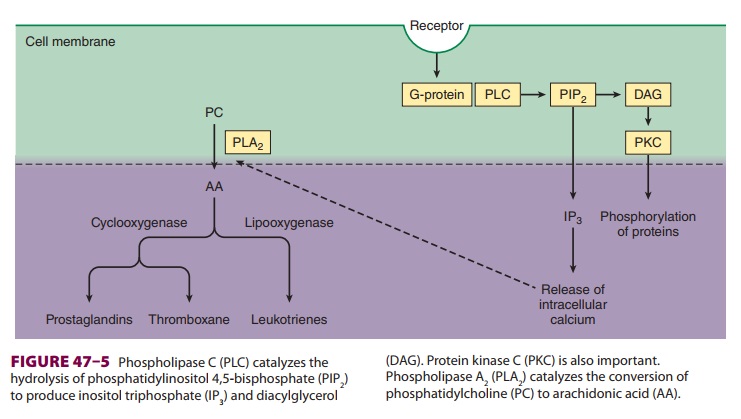
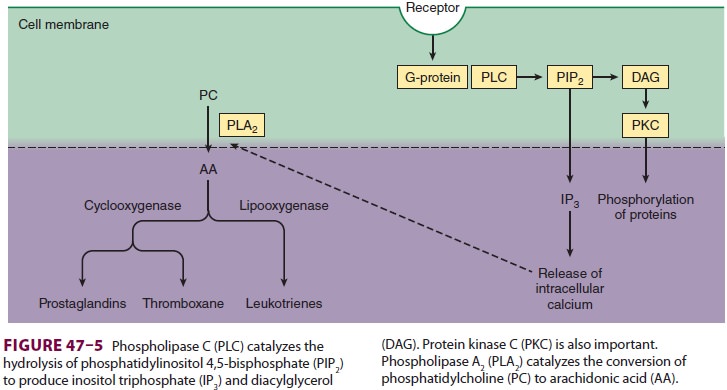
Prostaglandins are produced following tissue
damage by the action of phospholipase A 2 on phos-pholipids released from cell membranes to form arachidonic acid
(Figure 47–5). The cyclooxygen-ase
(COX) pathway then converts the latter into endoperoxides, which in turn are
transformed into prostacyclin and prostaglandin E2 (PGE2).
PGE2
directly activates free nerve endings, whereas pros-tacyclin potentiates the
edema from bradykinin. The lipoxygenase pathway converts arachidonic acid into
hydroperoxy compounds, which are sub-sequently converted into leukotrienes. The
role of the latter is not well defined, but they appear to potentiate certain
types of pain. Pharmacological agents such as acetylsalicylic acid (ASA, or
aspirin),
acetaminophen, and nonsteroidal
antiinflammatory drugs (NSAIDs) produce analgesia by inhibition of COX. The
analgesic effect of corticosteroids is likely the result of inhibition of
prostaglandin production through blockade of phospholipase A2 activation.
B. Secondary Hyperalgesia
Neurogenic inflammation, also called
secondary hyperalgesia, plays an important role in peripheral sensitization
following injury. It is manifested by the “triple response (of Lewis)” of a red
flush around the site of injury (flare), local tissue edema, and sensiti-zation
to noxious stimuli. Secondary hyperalgesia is primarily due to antidromic
release of substance P (and probably CGRP). Substance P degranulates his-tamine
and 5-HT, vasodilates blood vessels, causes tissue edema, and induces the
formation of leukotri-enes. The neural origin of this response is supported by
the following findings: (1) it can be produced by electrical stimulation of a
sensory nerve, (2) it is not observed in denervated skin, and (3) it is
diminished by injection of a local anesthetic. Capsaicin applied topically in a
gel, cream, or patch depletes substance P and diminishes neurogenic
inflammation, and is useful for some patients with postherpetic neuralgia.
Central Modulation of Pain
A. Facilitation
At least three mechanisms are responsible for central sensitization in
the spinal cord:
·
Wind-up and
sensitization of second-order neurons. WDR neurons increase their frequency of
discharge with the same repetitive stimuli and exhibit prolonged discharge,
even after afferent C fiber input has stopped.
·
Receptor fi eld expansion. Dorsal
horn neurons increase their receptive fields such that adjacent neurons become
responsive to stimuli (whether noxious or not) to which they were previously
unresponsive.
·
Hyperexcitability of
flexion reflexes. Enhancement of flexion reflexes is observed both
ipsilaterally and contralaterally.
Neurochemical mediators of central sensitiza-tion include substance P,
CGRP, vasoactive intestinal peptide (VIP), cholecystokinin (CCK), angiotensin,
and galanin, as well as the excitatory amino acids l-glutamate and l-aspartate.
These substances trig-ger changes in membrane excitability by interacting with
G protein–coupled membrane receptors on neurons (Figure 47–5).
Glutamate and aspartate play important roles in wind-up, via activation
of N-methyl-d-aspartate (NMDA) and
other receptor mechanisms, and in the induction and maintenance of central
sensitiza-tion. Activation of NMDA receptors also induces nitric oxide
synthetase, increasing formation of nitric oxide. Both prostaglandins and
nitric oxide facilitate the release of excitatory amino acids in the spinal
cord. Thus, COX inhibitors such as ASA and NSAIDs have important analgesic
actions in the spi-nal cord.
B. Inhibition
Transmission of nociceptive input in the spinal cord can be inhibited by
segmental activity in the cord itself, as well as by descending neural activity
from supraspinal centers.
1.Segmental inhibition—Activation of large affer-ent
fibers subserving sensation inhibits WDR neuron and spinothalamic tract activity. Moreover, activation of noxious stimuli in noncontiguous
parts of the body inhibits WDR neurons at other levels, which may explain why
pain in one part of the body inhibits pain in other parts. These two phenomena
support a “gate” theory for pain processing in the spinal cord.
Glycine and γ-aminobutyric acid (GABA) are amino acids that function as inhibitory
neurotrans-mitters and likely play an important role in segmen-tal inhibition
of pain in the spinal cord. Antagonism of glycine and GABA results in powerful
facilita-tion of WDR neurons and produces allodynia and hyperesthesia. There
are two subtypes of GABA receptors: GABA A, of which muscimol is an agonist, and GABAB, of which baclofen is an agonist. Seg-mental inhibition appears to be
mediated by GABA B receptor activity. The GABAA receptor functions as a Cl− channel, and benzodiazepines activate this chan-nel. Activation of
glycine receptors also increases Cl− conductance across neuronal cell membranes. The action of glycine is
more complex than that of GABA, because the former also has a facilitatory
(excitatory) effect on the NMDA receptor.
Adenosine also modulates nociceptive activity in the dorsal horn. At
least two receptors are known: A1, which inhibits adenyl
cyclase, and A2, which stimulates adenyl cyclase. The A1 receptor mediates adenosine’s
antinociceptive action. Methylxanthines can reverse this effect through
phosphodiesterase inhibition.
2. Supraspinal
inhibition—Several supraspinalstructures send fibers
down the spinal cord to inhib-it pain in the dorsal horn. Important sites of
origin for these descending pathways include the periaque-ductal gray,
reticular formation, and nucleus raphe magnus (NRM). Stimulation of the
periaqueduc-tal gray area in the midbrain produces widespread analgesia in
humans. Axons from these tracts act presynaptically on primary afferent neurons
and postsynaptically on second-order neurons (or in-terneurons). These pathways
mediate their antino-ciceptive action via α2-adrenergic, serotonergic, and opiate (µ, δ, and κ) receptor mechanisms.
The role of monoamines in pain inhibition explains the analge-sic efficacy of
antidepressants that block reuptake of catecholamines and serotonin.
Inhibitory adrenergic pathways originate pri-marily from the
periaqueductal gray area and the reticular formation. Norepinephrine mediates
this action via activation of presynaptic or postsynaptic α2receptors. At least part of the
descending inhibi-tion from the periaqueductal gray is relayed first to the NRM
and medullary reticular formation; sero-tonergic fibers from the NRM then relay
the inhi-bition to dorsal horn neurons via the dorsolateral funiculus.
The endogenous opiate system (primarily the
NRM and reticular formation) acts via methionine enkephalin, leucine
enkephalin, and β-endorphin, all of which
are antagonized by naloxone. These opioids act presynaptically to hyperpolarize
pri-mary afferent neurons and inhibit the release of substance P; they also
appear to cause some postsyn-aptic inhibition. Exogenous opioids preferentially
act postsynaptically on the second-order neurons or interneurons in the
substantia gelatinosa.
Related Topics