Chapter: Clinical Anesthesiology: Regional Anesthesia & Pain Management: Chronic Pain Management
Pain Pathways
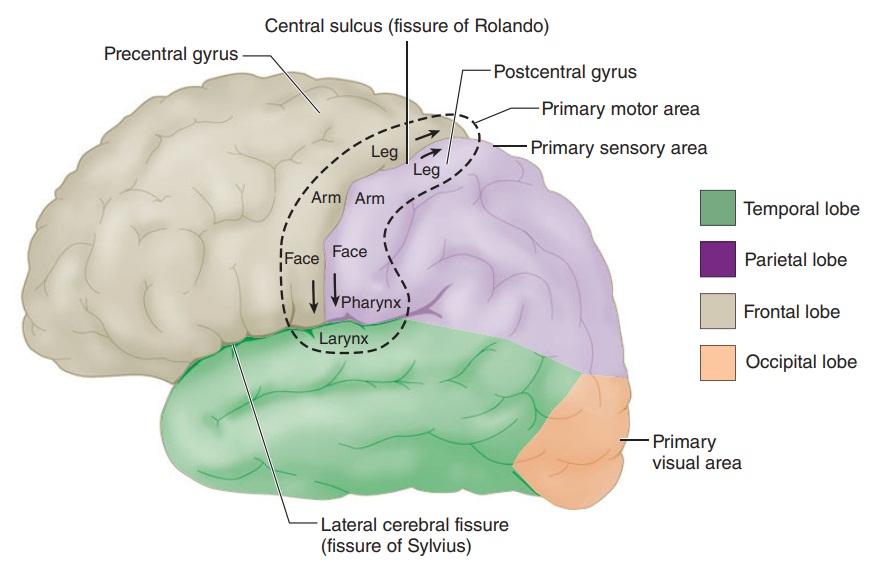
Anatomy & Physiology of Nociception
PAIN PATHWAYS
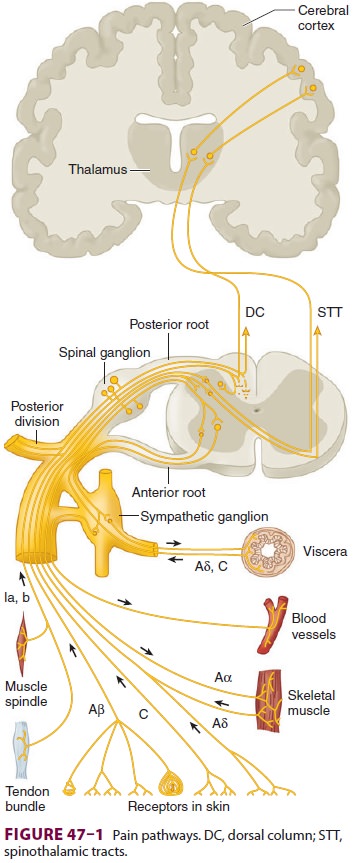
Pain is conducted along three neuronal
pathways that transmit noxious stimuli from the periphery to the cerebral
cortex (Figure 47–1). The cell bodies of
primary afferent neurons are located in the dorsal root ganglia, which lie in
the vertebral foramina at each spinal cord level. Each neuron has a single axon
that bifurcates, sending one end to the peripheral tissues it innervates and
the other into the dorsal horn of the spinal cord. In the dorsal horn, the
pri-mary afferent neuron synapses with a second-order neuron whose axon crosses
the midline and ascends in the contralateral spinothalamic tract to reach the
thalamus. Second-order neurons synapse in tha-lamic nuclei with third-order
neurons, which in turn send projections through the internal capsule and corona
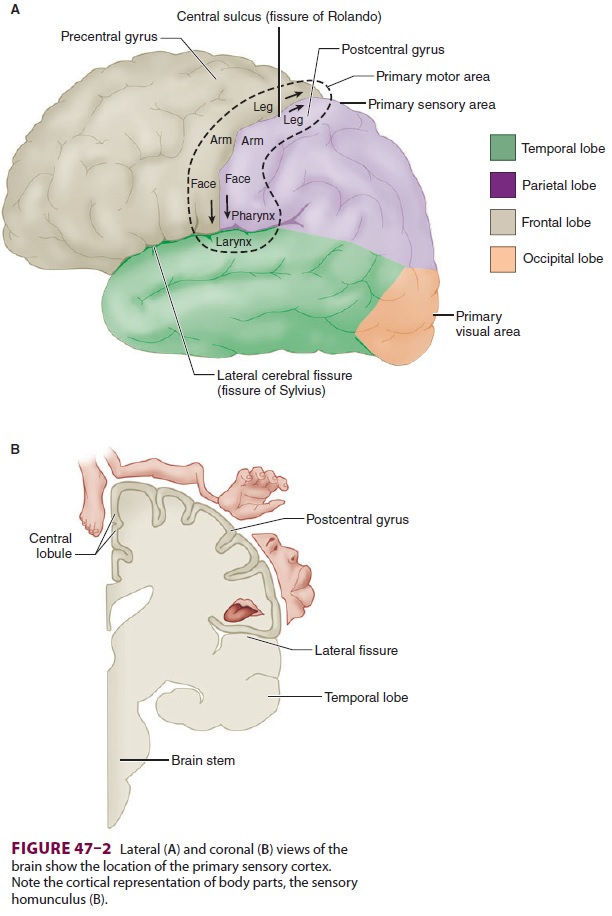
radiata to the postcentral gyrus of the cerebral cortex ( Figure
47–2).
First-Order Neurons
The majority of first-order neurons send the
proximal end of their axons into the spinal cord via the dorsal (sensory)
spinal root at each cervical, thoracic, lum-bar, and sacral level. Some
unmyelinated afferent (C) fibers have been shown to enter the spinal cord via
the ventral nerve (motor) root, accounting for obser-vations that some patients
continue to feel pain even after transection of the dorsal nerve root
(rhizotomy) and report pain following ventral root stimulation. Once in the
dorsal horn, in addition to synapsing with second-order neurons, the axons of
first-order neurons may synapse with interneurons, sympathetic neurons, and
ventral horn motor neurons.
Pain fibers originating from the head are carried by the trigeminal (V), facial (VII), glossopharyngeal (IX), and vagal (X) nerves. The gasserian ganglion contains the cell bodies of sensory fibers in the oph-thalmic, maxillary, and mandibular divisions of the trigeminal nerve. Cell bodies of first-order afferent neurons of the facial nerve are located in the genicu-late ganglion; those of the glossopharyngeal nerve lie in its superior and petrosal ganglia; and those of the vagal nerve are located in the jugular ganglion (somatic) and the ganglion nodosum (visceral). The proximal axonal processes of the first-order neurons in these ganglia reach the brainstem nuclei via their respective cranial nerves, where they synapse with second-order neurons in brainstem nuclei.
Second-Order Neurons
As afferent fibers enter the spinal cord,
they segre-gate according to size, with large, myelinated fibers becoming
medial, and small, unmyelinated fibers becoming lateral. Pain fibers may ascend
or descend one to three spinal cord segments in Lissauer’s tract before
synapsing with second-order neurons in the gray matter of the ipsilateral
dorsal horn. In many instances they communicate with second-order neurons
through interneurons.
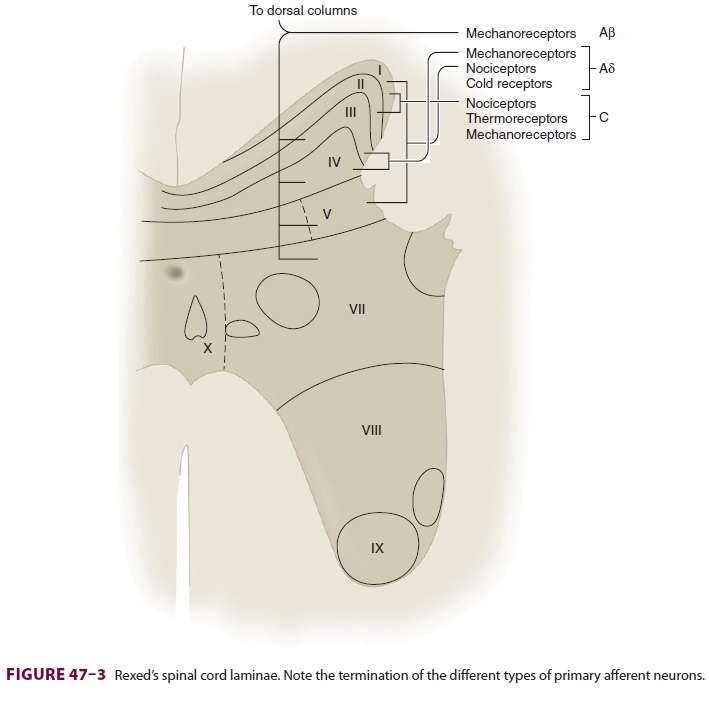
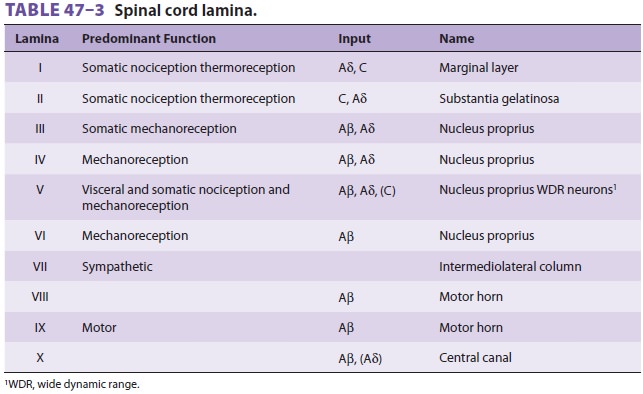
Spinal cord gray matter was divided by Rexed into 10 laminae (Figure 47–3 and Table 47–3). The first six laminae, which make up the dorsal horn, receive all afferent neural activity and represent the principal site of modulation of pain by ascending and descending neural pathways. Second-order neu-rons are either nociceptive-specific or wide dynamic range (WDR) neurons. Nociceptive-specific neu-rons serve only noxious stimuli, but WDR neurons also receive nonnoxious afferent input from Aβ, Aδ, and C fibers. Nociceptive-specific neurons are arranged somatotopically in lamina I and have discrete, somatic receptive fields; they are normally silent and respond only to high-threshold noxious stimulation, poorly encoding stimulus intensity. WDR neurons are the most prevalent cell type in the dorsal horn. Although they are found throughout the dorsal horn, WDR neurons are most abundant in lamina V. During repeated stimulation, WDR neurons characteristically increase their firing rate exponentially in a graded fashion (“wind-up”), even with the same stimulus intensity. They also have large receptive fields compared with nociceptive-specific neurons.
Most nociceptive C fibers send collaterals
to, or terminate on, second-order neurons in laminae I and II, and, to a lesser
extent, in lamina V. In contrast, nociceptive Aδ f ibers synapse mainly in laminae I and V, and, to a lesser degree, in
lamina X. Lamina I responds primarily to noxious (nociceptive) stimuli from
cutaneous and deep somatic tissues. Lamina II, also called the substantia
gelatinosa, contains many interneurons and is believed to play a major role in
processing and modulating nociceptive input from cutaneous nociceptors. It is
also of special interest because it is believed to be a major site of action
for opioids. Laminae III and IV primarily receive non-nociceptive sensory
input. Laminae VIII and IX make up the anterior (motor) horn. Lamina VII is the
intermediolateral column and contains the cell bodies of preganglionic
sympathetic neurons.
Visceral afferents terminate primarily in
lamina V, and, to a lesser extent, in lamina I. These two lami-nae represent
points of central convergence between somatic and visceral inputs. Lamina V
responds to both noxious and nonnoxious sensory input and receives both
visceral and somatic pain afferents. The phenomenon of convergence between
visceral and somatic sensory input is manifested clinically as referred pain
(see Table 47–2). Compared with somatic fibers, visceral nociceptive fibers are
fewer in number, more widely distributed, proportionately activate a larger
number of spinal neurons, and are not organized somatotopically.
A. The Spinothalamic Tract
The axons of most second-order neurons cross
the midline close to their dermatomal level of origin (at the anterior
commissure) to the contralateral side of the spinal cord before they form the
spinothalamic tract and send their fibers to the thalamus, the retic-ular
formation, the nucleus raphe magnus, and the periaqueductal gray. The
spinothalamic tract, which is classically considered the major pain pathway,
lies anterolaterally in the white matter of the spinal cord (Figure
47–4). This ascending tract can be divided into a
lateral and a medial tract. The lateral spino-thalamic (neospinothalamic) tract
projects mainly to the ventral posterolateral nucleus of the thalamus and
carries discriminative aspects of pain, such as location, intensity, and
duration. The medial spino-thalamic (paleospinothalamic) tract projects to the
medial thalamus and is responsible for mediating the autonomic and unpleasant
emotional percep-tions of pain. Some spinothalamic fibers also proj-ect to the
periaqueductal gray and thus may be an
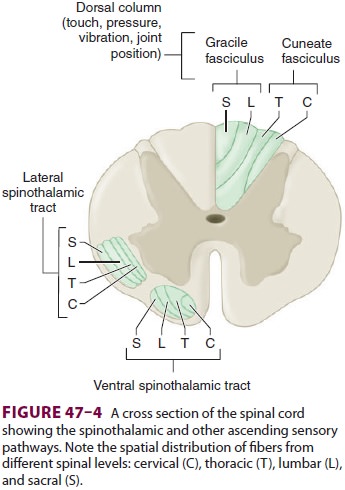
important link between the ascending and descend-ing pathways.
Collateral fibers also project to the reticular activating system and the
hypothalamus; these are likely responsible for the arousal response to pain.
B. Alternate Pain Pathways
As with epicritic sensation, pain fibers
ascend dif-fusely, ipsilaterally, and contralaterally; some patients continue
to perceive pain following ablation of the contralateral spinothalamic tract,
and there-fore other ascending pain pathways are also impor-tant. The
spinoreticular tract is thought to mediate arousal and autonomic responses to
pain. The spi-nomesencephalic tract may be important in activat-ing
antinociceptive, descending pathways, because it has some projections to the
periaqueductal gray. The spinohypothalamic and spinotelencephalic tracts
activate the hypothalamus and evoke emotional behavior. The spinocervical tract
ascends uncrossed to the lateral cervical nucleus, which relays the fibers to
the contralateral thalamus; this tract is likely a major alternative pathway
for pain. Lastly, some fibers in the dorsal columns (which mainly carry light
touch and proprioception) are responsive to pain; they ascend medially and
ipsilaterally.
C. Integration with the Sympathetic and Motor Systems
Somatic and visceral afferents are fully
integrated with the skeletal motor and sympathetic systems in the spinal cord,
brainstem, and higher centers. Afferent dorsal horn neurons synapse both
directly and indirectly with anterior horn motor neurons. These synapses are
responsible for the reflex mus-cle activity—whether normal or abnormal—that is
associated with pain. In a similar fashion, synapses between afferent
nociceptive neurons and sympa-thetic neurons in the intermediolateral column
result in reflex sympathetically mediated vasoconstriction, smooth muscle
spasm, and the release of catechol-amines, both locally and from the adrenal
medulla.
Third-Order Neurons
Third-order neurons are located in the
thalamus and send fibers to somatosensory areas I and II in the postcentral
gyrus of the parietal cortex and the superior wall of the sylvian fissure,
respectively. Per-ception and discrete localization of pain take place in these
cortical areas. Although most neurons from the lateral thalamic nuclei project
to the primary somatosensory cortex, neurons from the intralami-nar and medial
nuclei project to the anterior cingu-late gyrus and are likely involved in
mediating the suffering and emotional components of pain.
Related Topics