Chapter: Psychology: Motivation and Emotion
Physiological Aspects of Hunger and Eating
Physiological Aspects of Hunger and Eating
If
animals are temporarily deprived of food, they usually eat more later, to
return their bodies to the original weight. If they are force-fed extra food,
they later eat less. These
observations suggest that the animals have a caloric or body-weight set point that they seek to maintain.
In other words, animals do act as if they have an internal “appestat,”
maintaining a relatively steady weight, just as the hypothalamus, acting as the
body’s thermostat, maintains a relatively constant inner temperature.
Evidence
for some sort of internal set point also comes from the fact that when food is
freely available, animals usually eat just about the right amount to satisfy
their needs, while keeping their weight roughly constant. The “right amount”
here refers not to the volume of food, but to the number of calories in the
food—and hence, the metabolic energy it can provide. This was demonstrated in a
study many years ago in which researchers decreased the caloric levels of rats’
food by adding nonnutritive cellulose. The more diluted the food, the more the
rats ate, in a quantity that kept their total caloric intake roughly constant
(Adolph, 1947). Similar claims apply to humans, with the data indicating that
each of us seems to have a target weight that our bodies work homeostatically
to maintain. However, this set point is to some extent adjustable, and gradual
changes in one’s weight appear to alter the weight that is defended (Levitsky,
2002; Pinel, Assanand, & Lehman, 2000; Ruderman, 1986).
Evidence
for set points in humans comes from many sources, including the fact that crash
dieters usually return to their starting weight soon after they go off their
diets. Moreover, dieters do not lose nearly as much weight as we might expect
based on their reduced caloric intake. This is probably because the body
compensates for the caloric loss by reducing its metabolic rate (Guesbeck et
al., 2001). In other words, when the body gets less food, it responds by
burning fewer calories, defend-ing the set point weight. The consequence, of
course, is that eating less does not lead to weight loss.
THE ROLE OF THE LIVER
What
mechanisms maintain someone’s body weight at its set point? The answer involves
a number of internal signals, including signals that reflect the availability
of glucose (the sugar that serves as the body’s main fuel) in the blood.
Immediately
after a meal, glucose is plentiful. Some is used right away, but much is
converted to glycogen and various fatty acids, which are stored for later use.
When this stored energy is needed, the process is reversed, and the glycogen
and fatty acids are turned back into usable glucose. This conversion process is
managed by the liver, and the liver keeps other organs informed about the
direction in which the metabolic trans-action is going, from glucose to
glycogen or vice versa. If the supply of glucose exceeds the body’s demand, so
that the excess can be converted into glycogen and stored, the liver sends a
satiety signal and the animal stops eating. If the body’s demand for glu-cose
exceeds the supply, so that energy reserves are being used, the liver sends a
hunger signal and the animal eats (M. I. Friedman & Stricker, 1976; Russek,
1971).
Notice,
though, that this regulatory system must—like the thermoregulatory system—anticipate
the animal’s needs. Imagine that the liver only signaled the animal to eat when
glucose supplies were already low. Since it often takes time to locate food,
eat it, and then digest it, hours might elapse between the moment at which the
liver sends a “Need glucose!” signal and the time that the glucose finally
arrives. Nutrient supplies would be exhausted, and the animal could die. It’s
not surprising, therefore, that organisms have a well-defined mechanism for
avoiding this catastrophe. When an organism has not eaten for a while, the
level of glucose in the blood begins to drop. Before the level drops too far,
the liver takes action, drawing some glycogen out of storage and converting it
to glucose. As a result, the blood glucose level bounces back to normal. The
result of this sequence of events is an easily identifiable pattern—a grad-ual
drop in blood glucose, usually lasting many minutes, followed by a quick rise,
result-ing from the liver’s compensatory action.
This
slow-drop/quick-rise pattern means that the organism is drawing on its
reserves, which signals the need for more glucose. When this blood glucose
pattern occurs in rats, the animals start to eat (Campfield & Smith, 1990a,
b). When it occurs in humans, they say they are hungry (Campfield &
Rosenbaum, 1992).
OTHER CONTROL SIGNALS FOR FEEDING
The
liver is only one part of the body’s system for regulating food intake. The
hypothal-amus also contains cells that are sensitive to glucose levels in the
blood, and if these glucoreceptors are
damaged or disrupted, the result is ravenous eating (Miselis &Epstein,
1970).
Other
signals come from other parts of the body. The stomach walls, for example,
con-tain receptors sensitive to the nutrients dissolved in the digestive
juices. When these receptors signal to the brain that nutrient supplies are on
the way, the organism stops eating (Deutsch, Puerto, & Wang, 1978). Still
other signals come from the fatty tissues themselves. To understand the
importance of these signals, bear in mind that animals don’t eat just for the
moment. After all, they can’t be sure that food will be available the next time
they need energy, and so they must eat enough both to satisfy their current
needs and to store nutrients for later. This long-term store is provided by the
fat, or adi-pose cells, distributed throughout their body. These cells absorb
the fatty acids created by the liver and swell in the process. The longer-term
reserves then stand ready in case the animal’s glycogen supplies are exhausted.
If this happens, fatty acids drain from the adipose cells into the bloodstream
and are converted into glucose. Adipose tissue used to be regarded only as a
kind of inert storage,
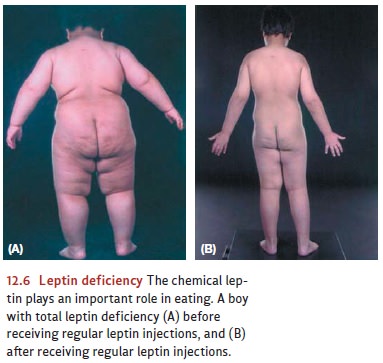
but
we now know that it plays a major role in governing hunger. Fat cells, when
full, secrete the chemical leptin
into the bloodstream, where it is sensed by receptors in several places in the
brain, includ-ing the hypothalamus (Bouret, Draper, & Simerly, 2004; Maffei
et al., 1995; McGregor et al., 1996; Pinto et al., 2004). Leptin seems to
signal that there is plenty of fat in storage and no need to add more, and it
may be one of the most important factors in governing an organism’s food intake
over the long term (Figure 12.6). Leptin appears to work by inhibiting the
actions of several other neuro-chemicals, such as neuropeptide Y (NPY), manufactured in the hypothalamus and the gut.
NPY itself turns out to be a powerful appetite stimulant (Gibbs, 1996; B. G.
Stanley, Magdalin, &Leibowitz, 1989), so leptin secretion from fat cells
seems to provide the negative feed-back that holds NPY levels in check.
HYPOTHALAMIC
CONTROL CENTERS
We
have now talked about many cues that signal an organism’s nutritional needs,
but what mechanism detects, and responds to, these cues? For years, the best
candidate was the hypothalamus. We have already mentioned that the hypothalamus
monitors blood sugar levels, but in addition, the hypothalamus has been
proposed as the receiving station for the body’s other eating-related cues, so
that the hypothalamus becomes, in effect, the main control center for feeding.
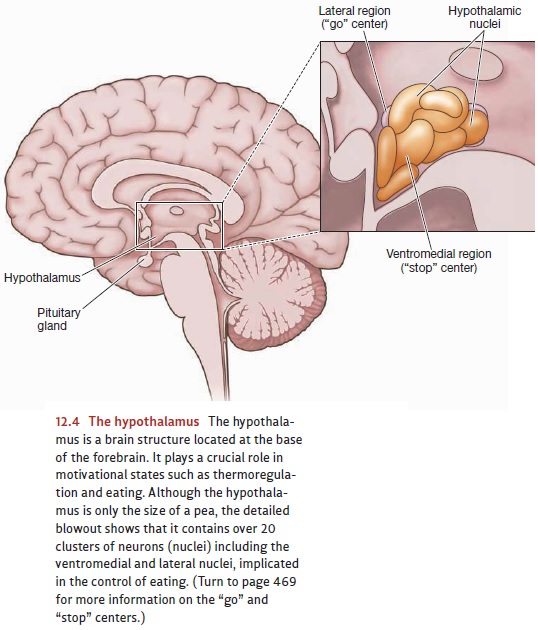
The dual-center theory proposed that
one part of the hypothalamus—the lateral region—served as the “go” center for
eating, while a different part—the ventromedial region—served as the “stop”
center (Figure 12.4).
Consistent
with this claim, damage to the lateral region of the hypothalamus seems to
disrupt the initiation of feeding. If
this region is lesioned, animals do not eat and will starve to death unless
force-fed. Conversely, damage to the ventromedial region dis-rupts circuits
that would ordinarily tell the animal when to stop feeding. Surgically induced lesions here cause rats to eat
voraciously, until they finally reach a weight three times as great as before
surgery (Figure 12.7). In humans, tumors in this hypothalamic region have the
same effect—leading to extreme obesity (Hoebel & Teitelbaum, 1976; N. E.
Miller, Bailey, & Stevenson, 1950; Teitelbaum & Epstein, 1962).

Subsequent
research has shown, however, that the mechanisms described in this theory are
only part of the story of how feeding is controlled. For example, lesions of
the ventromedial hypothalamus (the supposed “stop” center) have been found not
just to increase appetite (because the “stop” center is no longer functioning),
but also to increase the rate of fat storage (Stricker & Zigmond, 1976). In
addition, the lateral hypothalamus appears to be only one of the “go” centers
for feeding. This is indicated by the fact that the appetite stimulant NPY
exerts its strongest effects outside
the lateral hypothalamus (Leibowitz, 1991). These and other results indicate
that even though the hypothalamus is critical for the control of eating, other
mechanisms are also crucial, some specialized for short-term energy needs,
others for long-term storage.
WHY SO
MANY SIGNALS
?
We
have acknowledged a broad set of signals controlling when an organism starts
eating and when it stops, signals from the liver and from glucoreceptors in the
brain, signals from the stomach and from the adipose tissue. In truth, other
signals should be added to this list, including, of course, the sensory
qualities of the food itself. Thus, when we see a delicious-looking pastry or
smell hot, fresh popcorn, these sen-sory cues can make us feel hungry and cause
us to eat even if we are experiencing no caloric need.
Why
do we need so many cues? Part of the answer lies in the safety provided by
backup systems—so that if one system fails, the organism can still
self-regulate. And part of the answer is that different signals monitor
different aspects of our nutritional needs—some (such as leptin) keeping tabs
on our longer-term needs, and others (like cues from the stomach) signaling our
more immediate status and allowing us to deal with hour-by-hour variations in
our energy requirements.
The various cues also play different roles within the overall control of feeding. Some cues, like the sensory information from the food itself, directly signal the avail-ability of food in the environment. Other, less direct cues play their main role in creating a motivational state for the organism so that, broadly put, these cues lead the organism to feel hungry so it is motivated to seek food. Finally, some cues potentiate other cues—that is, they make the other cues more salient and more persuasive. In one study, for example, researchers recorded activity levels in cells in a waking mon-key’s hypothalamus (Mora, Rolls, & Burton, 1976; also Rolls, 1978). Those cells were activated when the animal was shown a peanut or banana, but only when the animal was hungry. In this fashion, the cues reflecting the animal’s internal state did not directly influence its behavior. Instead, these cues potentiated the sensory cues, so that the animal would be more likely to detect (and respond to) the immediate avail-ability of food when it needed nutrients.
Related Topics