Chapter: Medical Surgical Nursing: Assessment and Management of Patients With Diabetes Mellitus
Microvascular Complications and Diabetic Retinopathy - Long Term Complications of Diabetes
MICROVASCULAR
COMPLICATIONS AND DIABETIC RETINOPATHY
Although
macrovascular atherosclerotic changes are seen in both diabetic and nondiabetic
patients, the microvascular changes are unique to diabetes. Diabetic
microvascular disease (or micro-angiopathy) is characterized by capillary
basement membrane thickening. The basement membrane surrounds the endothelial
cells of the capillary. Researchers believe that increased blood glu-cose
levels react through a series of biochemical responses to thicken the basement
membrane to several times its normal thickness. Two areas affected by these
changes are the retina and the kidneys. Diabetic retinopathy is the leading
cause of blindness in people between 20 and 74 years of age in the United
States; it occurs in both type 1 and type 2 diabetes (ADA, Diabetic
Retinopathy, 2003). Similarly, about one in every four individu-als starting
dialysis has diabetic nephropathy.
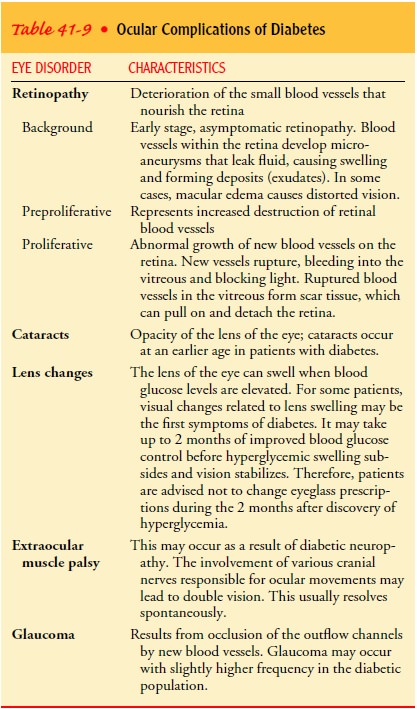
People
with diabetes are subject to multiple visual complica-tions (Table 41-9). The
eye pathology referred to as diabetic retinopathy is caused by changes in the
small blood vessels in the retina, the area of the eye that receives images and
sends infor-mation about the images to the brain (Fig. 41-9). It is richly
sup-plied with blood vessels of all kinds: small arteries and veins,
arterioles, venules, and capillaries. There are three main stages of
retinopathy: nonproliferative (background) retinopathy, prepro-liferative retinopathy,
and proliferative retinopathy.
Nearly
all patients with type 1 diabetes and more than 60% of patients with type 2
diabetes have some degree of retinopathy after 20 years (ADA, Diabetic
Retinopathy, 2003). Changes in the microvasculature include microaneurysms,
intraretinal hem-orrhage, hard exudates, and focal capillary closure. Although
most patients do not develop visual impairment, it can be devas-tating if it
occurs. A complication of nonproliferative retinopa-thy, macular edema, occurs
in approximately 10% of people with type 1 and type 2 diabetes and may lead to
visual distortion and loss of central vision.
An advanced form of background retinopathy, preprolifera-tive retinopathy, is considered a precursor to the more serious proliferative retinopathy. In preproliferative retinopathy, there are more widespread vascular changes and loss of nerve fibers.
Epidemiologic
evidence suggests that 10% to 50% of patients with preproliferative retinopathy
will develop proliferative retinopathy within a short time (possibly as little
as 1 year). As with back-ground retinopathy, if visual changes occur during the
preprolif-erative stage, they are usually caused by macular edema.
Proliferative
retinopathy represents the greatest threat to vision. Proliferative retinopathy
is characterized by the prolifera-tion of new blood vessels growing from the
retina into the vitreous. These new vessels are prone to bleeding. The visual
loss associated with proliferative retinopathy is caused by this vitreous
hemorrhage and/or retinal detachment. The vitreous is normally clear, allowing
light to be transmitted to the retina. When there is a hemorrhage, the vitreous
becomes clouded and cannot trans-mit light, resulting in loss of vision.
Another consequence of vit-reous hemorrhage is that resorption of the blood in
the vitreous leads to the formation of fibrous scar tissue. This scar tissue
may place traction on the retina, resulting in retinal detachment and
subsequent visual loss.
Clinical Manifestations
Retinopathy
is a painless process. In nonproliferative and pre-proliferative retinopathy,
blurry vision secondary to macular edema occurs in some patients, although many
patients are asymp-tomatic. Even patients with a significant degree of
proliferative retinopathy and some hemorrhaging may not experience major visual
changes. However, symptoms indicative of hemorrhaging include floaters or
cobwebs in the visual field, or sudden visual changes including spotty or hazy
vision, or complete loss of vision.
Assessment and Diagnostic Findings
Diagnosis
is by direct visualization with an ophthalmoscope or with a technique known as
fluorescein angiography. Fluorescein angiography can document the type and activity
of the retinopa-thy. Dye is injected into an arm vein and is carried to various
parts of the body through the blood, but especially through the vessels of the
retina of the eye. This technique allows the ophthalmolo-gist, using special
instruments, to see the retinal vessels in bright detail and gives useful
information that cannot be obtained with just an ophthalmoscope.
Side
effects of this diagnostic procedure may include nausea during the dye
injection; yellowish, fluorescent discoloration ofthe skin and urine lasting 12
to 24 hours; and occasional allergic reactions, usually manifested by hives or
itching. Generally, how-ever, it is a safe diagnostic procedure. Patient
preparation includes explaining:
· The steps of the
procedure
· The fact that the
procedure is painless
· The potential side
effects
· The type of information
the technique can provide
· That the flash of the
camera may be slightly uncomfortable for a short time
Medical Management
The
first focus of management is on primary and secondary pre-vention. The results
of the DCCT study demonstrated that maintenance of blood glucose to a normal or
near-normal level in type 1 diabetes through intensive insulin therapy and
patient education decreased the risk for development of retinopathy by 76% when
compared with conventional therapy in patients with-out preexisting
retinopathy. The progression of retinopathy was decreased by 54% in patients
with very mild to moderate non-proliferative retinopathy at the time of
initiation of treatment. Similarly, the UKPDS study demonstrated a reduced risk
of retinopathy in type 2 diabetes with better control of blood glu-cose levels
(ADA, Diabetic Retinopathy, 2003).
For
advanced cases, the main treatment of diabetic retinopa-thy is argon laser
photocoagulation. The laser treatment destroys leaking blood vessels and areas
of neovascularization. For patients at increased risk for hemorrhaging,
panretinal photocoagulation may significantly reduce the rate of progression to
blindness. Pan-retinal photocoagulation involves the systematic application of
multiple (more than 1,000) laser burns throughout the retina (ex-cept in the
macular region). This stops the widespread growth of new vessels and
hemorrhaging of damaged vessels. The role of “mild” panretinal photocoagulation
(with only a third to a half as many laser burns) in the early stages of
proliferative retinopa-thy or in patients with preproliferative changes is
being investi-gated. For macular edema, focal photocoagulation is used to apply
smaller laser burns to specific areas of microaneurysms in the macular region.
This may reduce the rate of visual loss from macular edema by 50% (ADA,
Diabetic Retinopathy, 2003).
Photocoagulation
treatments are usually performed on an out-patient basis, and most patients can
return to their usual activi-ties by the next day. For some patients,
limitations may be placed on activities involving weight bearing or bearing
down. For most patients, the treatment does not cause intense pain, although
they may report varying degrees of discomfort. Usually an anesthetic eye drop
is all that is needed during the treatment. A few patients may experience
slight visual loss, loss of peripheral vision, or im-pairments in adaptation to
the dark. For most patients, however, the risk of slight visual changes from
the laser treatment itself is much less than the potential for loss of vision
from progression of retinopathy.
When a
major hemorrhage into the vitreous occurs, the vitre-ous fluid becomes mixed
with blood and prevents light from pass-ing through the eye; this can cause
blindness. A vitrectomy is a surgical procedure in which vitreous humor filled
with blood or fibrous tissue is removed with a special drill-like instrument
and replaced with saline or another liquid. A vitrectomy is performed on
patients who already have visual loss and in whom the vitre-ous hemorrhage has
not cleared on its own after 6 months. The purpose is to restore useful vision;
recovery to near-normal vision is not usually expected. Other strategies that
may slow the pro-gression of diabetic retinopathy include:
·
Control of hypertension
·
Control of blood glucose
·
Cessation of smoking
Nursing Management
Nursing
management of patients with diabetic retinopathy or other eye disorders
involves implementing the individual plan of care and providing patient
education. Education focuses on pre-vention through regular ophthalmologic
examinations and blood glucose control and self-management of eye care
regimens. The effectiveness of early diagnosis and prompt treatment is
empha-sized in teaching the patient and family. If vision loss occurs, nursing
care must also address the patient’s adjustment to im-paired vision and use of
adaptive devices for diabetes self-care as well as activities of daily living.
PROMOTING HOME AND COMMUNITY-BASED CARE
Teaching Patients Self-Care.
In all forms
of therapy for retinopa-thy, something is destroyed in the process of saving
vision, and the facts must be presented to the patient and family as honestly
as possible. The course of the retinopathy may be long and stress-ful. In
teaching and counseling the patient, it is important to stress the following:
· Retinopathy may appear
after many years of diabetes, and its appearance does not necessarily mean that
the diabetes is on a downhill course.
· The odds for maintaining
vision are in the patient’s favor, especially with adequate control of glucose
levels and blood pressure.
· Frequent eye
examinations are the best way to preserve vision, because they allow for the
detection of any re-tinopathy.
Some
additional points to keep in mind when the patient with diabetes has some type
of visual impairment include the following:
· Visual impairment can be
a shock. The person’s response to vision loss depends on personality,
self-concept, and coping mechanisms.
· As in any loss,
acceptance of blindness by the patient occurs in stages; some patients may
learn to accept blindness in a rather short period, and others may never do so.
· Although retinopathy
occurs bilaterally, the severity may differ in the two eyes.
· Many of the chronic
complications of diabetes occur si-multaneously. For example, a patient who is
blind due to diabetic retinopathy may also have peripheral neuropathy and may
experience impairment of manual dexterity and tactile sensation.
Continuing Care.
Continuing
care for the patient with impairedvision due to diabetic changes depends on the
severity of the im-pairment and the effectiveness of the patient’s coping in
response to the impairment. The importance of careful diabetes manage-ment is
emphasized as one means of slowing the progression of visual changes. The
patient is reminded of the need to see the ophthalmologist regularly. If eye
changes are progressive and un-relenting, the patient needs to be prepared for
inevitable blind-ness. Therefore, consideration is given to making referrals
for teaching the patient Braille and for training with a guide dog. Re-ferral
to state agencies should be made to ensure that the patient receives services
for the blind. Family members are also taught how to assist the patient to
remain as independent as possible de-spite decreasing visual acuity.
Referral
for home care may be indicated for some patients, particularly those who live
alone, those not coping well, and those who have other health problems or
complications of diabetes that may interfere with their ability to perform
self-care. During home visits, the nurse can assess the patient’s home
environment and ability to manage diabetes despite visual impairments.
Related Topics