Chapter: Medical Surgical Nursing: Assessment and Management of Patients With Diabetes Mellitus
Diabetes Management: Pharmacologic Therapy
PHARMACOLOGIC
THERAPY
As
stated earlier, insulin is secreted by the beta cells of the islets of
Langerhans and works to lower the blood glucose level after meals by
facilitating the uptake and utilization of glucose by muscle, fat, and liver
cells. In the absence of adequate insulin, pharmacologic therapy is essential.
Insulin Therapy and Insulin Preparations
Because
the body loses the ability to produce insulin in type 1 di-abetes, exogenous
insulin must be administered for life. In type 2 diabetes, insulin may be
necessary on a long-term basis to control glucose levels if diet and oral
agents fail. In addition, some pa-tients in whom type 2 diabetes is usually
controlled by diet alone or by diet and an oral agent may require insulin
temporarily during illness, infection, pregnancy, surgery, or some other
stress-ful event. In many cases, insulin injections are administered two or
more times daily to control the blood glucose level. Because the insulin dose
required by the individual patient is determined by the level of glucose in the
blood, accurate monitoring of blood glucose levels is essential; thus, SMBG has
become a cornerstone of insulin therapy. A number of insulin preparations are
available. They vary according to three main characteristics: time course of
action, species (source), and manufacturer.
TIME COURSE OF ACTION
Insulins
may be grouped into several categories based on the onset, peak, and duration
of action (Table 41-3). Human insulin preparations have a shorter duration of
action than insulin from animal sources because the presence of animal proteins
triggers an immune response that results in the binding of animal insulin, which
slows its availability.
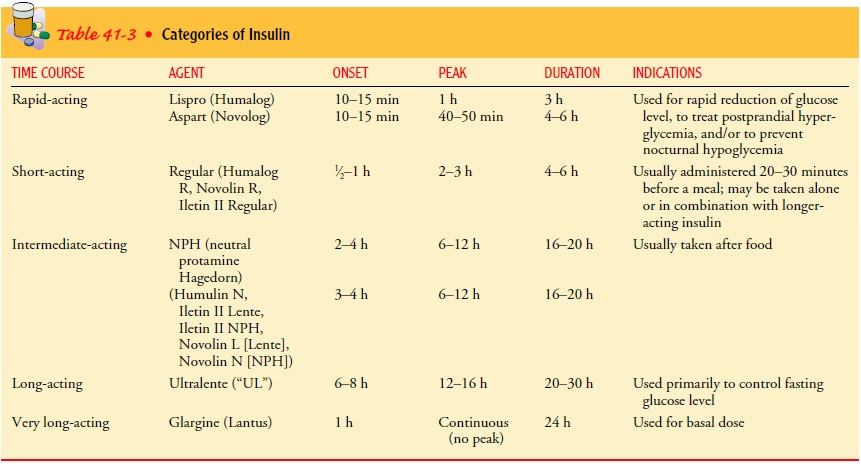
Rapid-acting insulins such as insulin lispro (Humalog) and in-sulin aspart (Novolog) are blood glucose-lowering agents that produce
a more rapid effect that is of shorter duration than reg-ular insulin. These
insulins have an onset of 5 to 15 minutes, a peak action of 1 hour after
injection, and a duration of 2 to 4 hours. Because of their rapid onset,
patients should be instructed to eat no more than 5 to 15 minutes after
injection. Because of the short duration of action of these insulin analogs,
patients with type 1 diabetes and some patients with type 2 or gestational
diabetes also require a long-acting insulin to maintain glucose control. Basal
insulin is necessary to maintain blood glucose levels irrespective of meals. A
constant level of insulin is required at all times. Intermediate-acting
insulins function as basal insulins but may have to be split into two
injections to achieve 24-hour coverage.
Short-acting
insulins, called regular insulin (marked R on the bottle), have an onset of 30
minutes to 1 hour; peak, 2 to 3 hours; and duration, 4 to 6 hours. Regular
insulin is a clear solution and is usually administered 20 to 30 minutes before
a meal, either alone or in combination with a longer-acting insulin. Humulin R,
Iletin Regular, and Novolin R are examples of regular insulin.
Intermediate-acting
insulins, called NPH insulin (neutral pro-tamine Hagedorn) or Lente insulin,
have an onset of 3 to 4 hours; peak, 4 to 12 hours; and duration, 16 to 20
hours. Intermediate-acting insulins, which are similar in their time course of
action, ap-pear white and cloudy. If NPH or Lente insulin is taken alone, it is
not crucial that it be taken 30 minutes before the meal. It is im-portant,
however, for the patient to eat some food around the time of the onset and peak
of these insulins. Humulin N, Iletin NPH, and Novolin N are examples of NPH
insulins; Humulin L, Iletin L, and Novolin L are examples of Lente insulins.
Long-acting
insulins, called Ultralente insulin, are sometimes referred to as peakless
insulins because they tend to have a long, slow, sustained action rather than
sharp, definite peaks in action. The onset of long-acting human insulin is 6 to
8 hours; peak, 12 to 16 hours; and duration, 20 to 30 hours.
“Peakless”
basal insulin, insulin glargine (Lantus), is approved for use as a basal
insulin—that is, the insulin is absorbed very slowly over 24 hours and can be
given once a day. Because the insulin is in a suspension with a pH of 4, it
cannot be mixed with other insulins because this would cause precipitation. It
is given once a day at bedtime.
In the
future, “inhaled insulin” may be approved for use. This type of insulin is in
the form of a very fine powder, which is in-haled through a device similar to
that used to administer asthma medications. The patient’s program would consist
of a “basal” rate of insulin such as glargine supplemented by an inhaled dose
before each meal.
The
nurse may find that different sources list differing num-bers of hours for the
onset, peak, and duration of action of the main types of insulin, and patients’
responses may vary (ie, larger doses prolong onset, duration, and peak). The
nurse should focus on which meals—and snacks—are being “covered” by which
in-sulin doses. In general, the rapid- and short-acting insulins are ex-pected
to cover the rise in glucose levels after meals, immediately after the
injection; the intermediate-acting insulins are expected to cover subsequent
meals; and the long-acting insulins provide a relatively constant level of
insulin and act as a basal insulin.
SPECIES (SOURCE)
In the
past, all insulins were obtained from beef (cow) and pork (pig) pancreases.
“Human insulins” are now widely available. They are produced by recombinant DNA
technology and have largely replaced insulin from animal sources (ADA, Insulin
Administration, 2003).
MANUFACTURER
The
two manufacturers of insulin in the United States are Eli-Lilly and Novo
Nordisk. The insulins made by the different companies are usually
interchangeable, provided the concentration (eg, U-100), species (eg, human),
and type (eg, NPH) of insulin are the same. Human insulins made by different
companies have different brand names. Therefore, a patient taking 20 units
human NPH insulin may be using either Humulin N or Novolin N.
Insulin Regimens
Insulin
regimens vary from one to four injections per day. Usually there is a
combination of a short-acting insulin and a longer-acting insulin. The normally
functioning pancreas continuously secretes small amounts of insulin during the
day and night. In ad-dition, whenever blood glucose rises after ingestion of
food, there is a rapid burst of insulin secretion in proportion to the
glucose-raising effect of the food. The goal of all but the simplest,
one-injection insulin regimens is to mimic this normal pattern of insulin
secretion in response to food intake and activity patterns. Table 41-4
describes several insulin regimens and the advantages and disadvantages of
each.
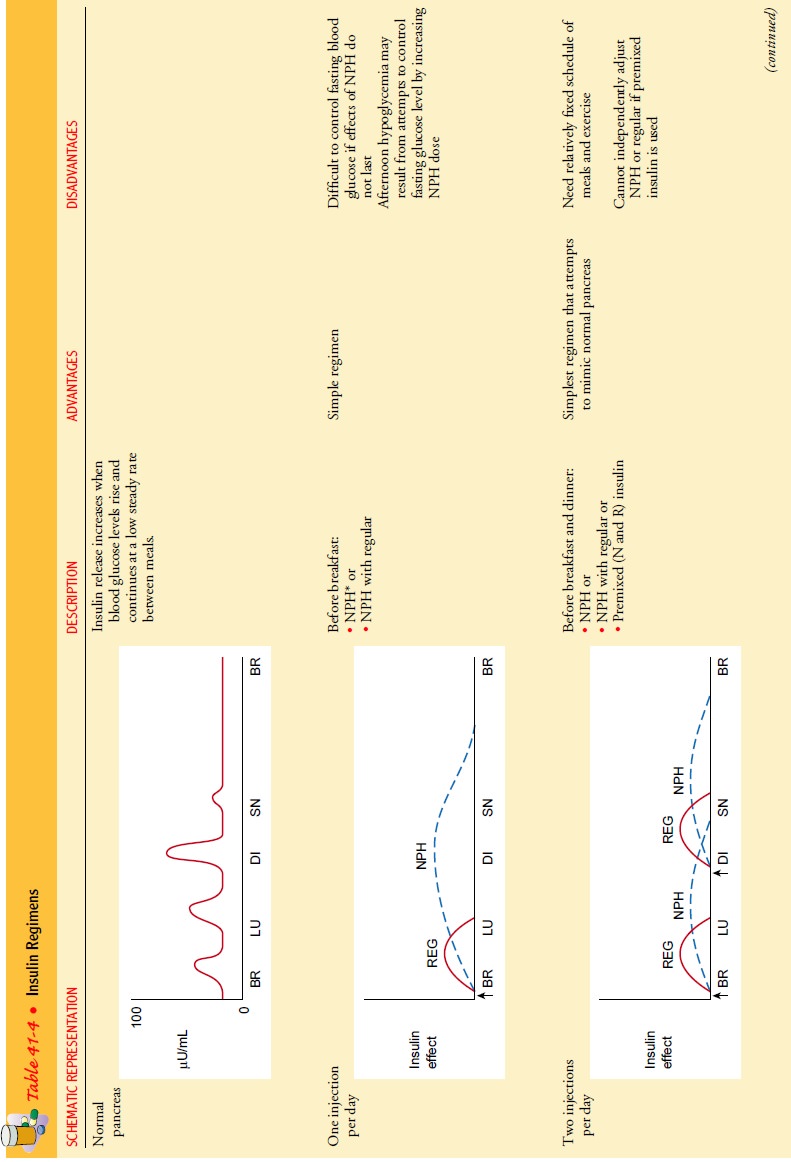
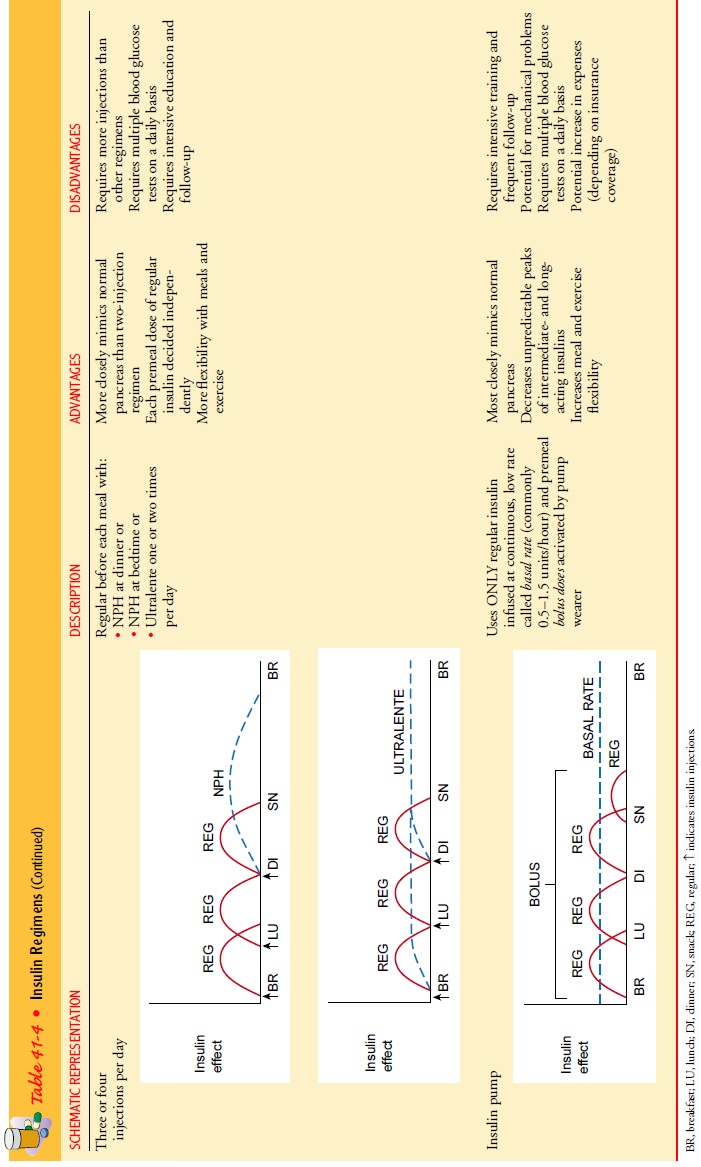
Patients
can learn to use SMBG results and carbohydrate counting to vary the insulin
doses. This allows patients more flex-ibility in the timing and content of
meals and exercise periods. However, complex insulin regimens require a strong
level of commitment, intensive education, and close follow-up by the health
care team. In addition, patients aiming for normal blood glucose levels run the
risk of more hypoglycemic reactions.
The
type of regimen used by any particular patient varies. For example, patient
knowledge, willingness, goals, health status, and finances all may affect
decisions regarding insulin treatment. In addition, the physician’s philosophy
about blood glucose control and the availability of equipment and support staff
may influence decisions regarding insulin therapy. There are two general
ap-proaches to insulin therapy: conventional and intensive.
CONVENTIONAL REGIMEN
One
approach is to simplify the insulin regimen as much as pos-sible, with the aim
of avoiding the acute complications of diabetes (hypoglycemia and symptomatic
hyperglycemia). With this type of simplified regimen (eg, one or more
injections of a mixture of short- and intermediate-acting insulins per day),
patients may fre-quently have blood glucose levels well above normal. The
excep-tion is the patient who never varies meal patterns and activity levels.
This approach would be appropriate for the terminally ill, the frail elderly
with limited self-care abilities, or any patient who is completely unwilling or
unable to engage in the self-management activities that are part of a more
complex insulin regimen.
INTENSIVE REGIMEN
The
second approach is to use a more complex insulin regimen to achieve as much
control over blood glucose levels as is safe and practical. The results of the
landmark DCCT study (1993) and the UKPDS study (1998) have demonstrated that
maintaining blood glucose levels as close to normal as possible prevents or
slows the progression of long-term diabetic complications. Another reason for
using a more complex insulin regimen is to allow pa-tients more flexibility to
change their insulin doses from day to day in accordance with changes in their
eating and activity pat-terns, with stress and illness, and as needed for
variations in the prevailing glucose level.
Although
the DCCT found that intensive treatment (three or four injections of insulin
per day) reduced the risk of complica-tions, not all people with diabetes are
candidates for very tight control of blood glucose. The risk for severe
hypoglycemia was increased threefold in patients receiving intensive treatment
in the DCCT (ADA, Implications of the Diabetes Control and Complications Trial,
2003). Those who may not be candidates include patients with:
•
Nervous system disorders rendering them unaware of
hypo-glycemic episodes (eg, those with autonomic neuropathy)
•
Recurring severe hypoglycemia
•
Irreversible diabetic complications, such as
blindness or end-stage renal disease
•
Cerebrovascular and/or cardiovascular disease
•
Ineffective self-care skills
An
exception is the patient who has received a kidney trans-plant because of
nephropathy and chronic renal failure; this pa-tient should be on an intensive
regimen to preserve function of the new kidney.
The
patient needs to be involved in the decision regarding which insulin regimen to
use. Patients need to compare the po-tential benefits of different regimens
with the potential costs (eg, time involved, number of injections or finger
sticks for glu-cose testing, amount of record-keeping). There are no set
guide-lines as to which insulin regimen should be used for which patients. It
must not be assumed that an elderly patient or a patient with visual impairment
should automatically be given a simplified regimen. Likewise, it must not be
assumed that all people will want to be involved in a complex treatment
regimen. Nurses play an important role in educating patients about the
different ap-proaches to insulin therapy. Nurses should refer patients to
dia-betes specialists or diabetes education centers, when available, for
further training and education in the various insulin treatment regimens.
Complications of Insulin Therapy
LOCAL ALLERGIC REACTIONS
A
local allergic reaction (redness, swelling, tenderness, and in-duration or a 2-
to 4-cm wheal) may appear at the injection site 1 to 2 hours after the insulin
administration. These reactions, which usually occur during the beginning
stages of therapy and disappear with continued use of insulin, are becoming
rare be-cause of the increased use of human insulins. The physician may
prescribe an antihistamine to be taken 1 hour before the injection if such a
local reaction occurs.
SYSTEMIC ALLERGIC REACTIONS
Systemic
allergic reactions to insulin are rare. When they do occur, there is an
immediate local skin reaction that gradually spreads into generalized urticaria
(hives). The treatment is de-sensitization, with small doses of insulin
administered in gradu-ally increasing amounts using a desensitization kit.
These rare reactions are occasionally associated with generalized edema or
anaphylaxis.
INSULIN LIPODYSTROPHY
Lipodystrophy
refers to a localized reaction, in the form of either lipoatrophy or
lipohypertrophy, occurring at the site of insulin injections. Lipoatrophy is
loss of subcutaneous fat and appears as slight dimpling or more serious pitting
of subcutaneous fat. The use of human insulin has almost eliminated this
disfiguring complication.
Lipohypertrophy, the development of fibrofatty masses at the injection site, is caused by the repeated use of an injection site. If insulin is injected into scarred areas, absorption may be delayed.
This
is one reason that rotation of injection sites is so important. The patient
should avoid injecting insulin into these areas until the hypertrophy
disappears.
INSULIN RESISTANCE
Most
patients at one time or another have some degree of insulin resistance. This
may occur for various reasons, the most common being obesity, which can be
overcome by weight loss. Clinical in-sulin resistance has been defined as a
daily insulin requirement of 200 units or more. In most diabetic patients
taking insulin, im-mune antibodies develop and bind the insulin, thereby
decreas-ing the insulin available for use. All animal insulins, as well as
human insulins to a lesser degree, cause antibody production in humans.
Very
few resistant patients develop high levels of antibodies. Many of these
patients have a history of insulin therapy inter-rupted for several months or
more. Treatment consists of admin-istering a more concentrated insulin
preparation, such as U500, which is available by special order. Occasionally,
prednisone is needed to block the production of antibodies. This may be
fol-lowed by a gradual reduction in insulin requirement. Therefore, patients
need to monitor themselves for hypoglycemia.
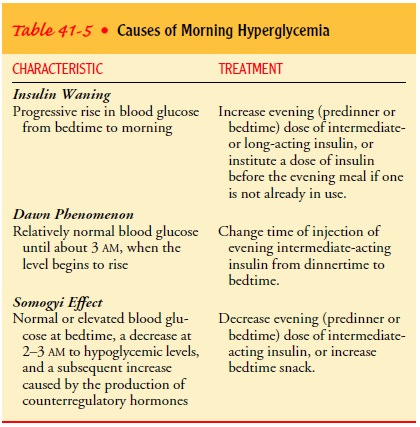
MORNING HYPERGLYCEMIA
An
elevated blood glucose level upon arising in the morning may be caused by an
insufficient level of insulin due to several causes: the dawn phenomenon, the
Somogyi effect, or insulin waning. The dawn phenomenon is characterized by a
relatively normal blood glucose level until approximately 3 a.m., when blood
glucose levels begin to rise. The phenomenon is thought to result from
nocturnal surges in growth hormone secretion that create a greater need for
insulin in the early morning hours in patients with type 1 diabetes. It must be
distinguished from insulin waning (the progressive increase in blood glucose
from bedtime to morning) or the Somogyi effect (nocturnal hypo-glycemia
followed by rebound hyperglycemia). Insulin waning is frequently seen if the
evening NPH dose is administered be-fore dinner and is prevented by moving the
evening dose of NPH insulin to bedtime.
It may
be difficult to tell from the patient’s history which of these causes is
responsible for morning hyperglycemia. To deter-mine the cause, the patient
must be awakened once or twice during the night to test blood glucose levels.
Testing the blood glucose level at bedtime, at 3 a.m., and on awakening
provides information that can be used in making adjustments in insulin to avoid
morning hyperglycemia caused by the dawn phenomenon. Table 41-5 summarizes the
differences among insulin waning, the dawn phenomenon, and the Somogyi effect.
Alternative Methods of Insulin Delivery
INSULIN PENS
These devices use small (150- to 300-unit) prefilled insulin car-tridges that are loaded into a penlike holder. A disposable needle is attached to the device for insulin injection. Insulin is delivered by dialing in a dose or pushing a button for every 1- or 2-unit in-crement administered. People using these devices still need to in-sert the needle for each injection; however, they do not need to carry insulin bottles or to draw up insulin before each injection. These devices are most useful for patients who need to inject only one type of insulin at a time (eg, premeal regular insulin three times a day and bedtime NPH insulin) or who can use the pre-mixed insulins. These pens are convenient for those who administer insulin before dinner if eating out or traveling. They are also useful for patients with impaired manual dexterity, vision, or cog-nitive function that makes the use of traditional syringes difficult.
JET INJECTORS
As an
alternative to needle injections, jet injection devices deliver insulin through
the skin under pressure in an extremely fine stream. These devices are more
expensive than other alternative devices mentioned above and require thorough
training and su-pervision when first used. In addition, patients should be
cau-tioned that absorption rates, peak insulin activity, and insulin levels may
be different when changing to a jet injector. (Insulin administered by jet
injector is usually absorbed faster.) Bruising has occurred in some patients
with use of the jet injector.
INSULIN PUMPS
Continuous subcutaneous insulin infusion involves the use
ofsmall, externally worn devices that closely mimic the functioning of the
normal pancreas (ADA, Continuous Subcutaneous Insulin Infusion, 2003). Insulin
pumps contain a 3-mL syringe attached to a long (24- to 42-in), thin,
narrow-lumen tube with a needle or Teflon catheter attached to the end (Figs.
41-4 and 41-5). The patient inserts the needle or catheter into the
subcutaneous tissue (usually on the abdomen) and secures it with tape or a
transparent dressing. The needle or catheter is changed at least every 3 days.
The pump is then worn either on a belt or in a pocket. Some women keep the pump
tucked into the front or side of the bra or wear it on a garter belt on the
thigh.
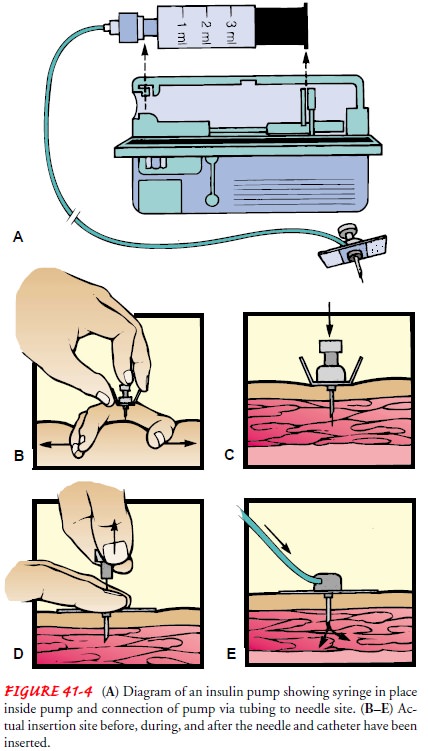
The rapid-acting lispro insulin is used in the insulin pump and is delivered at a basal rate and as a bolus with meals. A con-tinuous basal rate of insulin is typically 0.5 to 2.0 units/hour, depending on the patient’s needs. A bolus dose of insulin is delivered before each meal when the patient activates the pump (by pushing buttons). The patient determines the amount of in-sulin to infuse based on blood glucose levels and anticipated food intake and activity level. Advantages of insulin pumps include increased flexibility in lifestyle (in terms of timing and amount of meals, exercise, and travel) and, for many patients, improved blood glucose control.
A
disadvantage of insulin pumps is that unexpected disrup-tions in the flow of
insulin from the pump may occur if the tubing or needle becomes occluded, if
the supply of insulin runs out, or if the battery is depleted, increasing the
risk of DKA. Effective teaching and a knowledgeable patient can minimize this
risk. Another disadvantage is the potential for infection at needle in-sertion
sites. Hypoglycemia may occur with insulin pump ther-apy; however, this is
usually related to the lowered blood glucose levels many patients achieve
rather than to a specific problem with the pump itself. The tight diabetic
control associated with using an insulin pump may increase the incidence of
hypoglycemia un-awareness because of the very gradual decline in serum glucose
level from levels greater than 70 mg/dL (3.9 mmol/L) to those less than 60
mg/dL (3.3 mmol/L).
Some
patients find that wearing the pump for 24 hours each day is an inconvenience.
However, the pump can easily be dis-connected, per patient preference, for
limited periods (eg, for showering, exercise, or sexual activity).
Insulin
pump candidates must be willing to assess blood glu-cose levels multiple times
daily while on pump therapy. In addi-tion, they must be psychologically stable
and open about having diabetes, because the insulin pump is often a visible
sign to others and a constant reminder to the patient that he or she has
diabetes. Most important, patients using insulin pumps must have exten-sive
education in the use of the insulin pump and in self-management of blood
glucose and insulin doses. They must work closely with a team of health care
professionals who are experi-enced in insulin pump therapy—specifically, a
diabetologist/ endocrinologist, a dietitian, and a certified diabetes educator.
Many
insurance policies cover the cost of pump therapy; if it is not covered, the
extra expense of the pump and associated sup-plies may be a deterrent for some
patients. Medicare now covers insulin pump therapy for the patient with type 1
diabetes.
IMPLANTABLE AND INHALANT INSULIN DELIVERY
Research
into mechanical delivery of insulin has involved im-plantable insulin pumps
that can be externally programmed ac-cording to blood glucose test results.
Clinical trials with these devices are continuing. In addition, there is
research into the de-velopment of implantable devices that both measure the
blood glucose level and deliver insulin as needed. Methods of adminis-tering
insulin by the oral route (oral spray or capsule), skin patch, and inhalation
are undergoing intensive study.
TRANSPLANTATION OF PANCREATIC CELLS
Transplantation
of the whole pancreas or a segment of the pan-creas is being performed on a
limited population (mostly diabetic patients receiving kidney transplantations
simultaneously). One main issue regarding pancreatic transplantation is
weighing the risks of antirejection medications against the advantages of
pancreas transplantation. Another approach under investigation is the
implantation of insulin-producing pancreatic islet cells (ADA, Pancreas
Transplantation for Patients With Type 1 Dia-betes, 2003). This latter approach
involves a less extensive surgi-cal procedure and a potentially lower incidence
of immunogenic problems. However, thus far, independence from exogenous
in-sulin has been limited to 2 years after transplantation of islet cells. A
recent study of patients with islet cell
transplants using less toxic antirejection drugs has shown promise (Shapiro
et al., 2000).
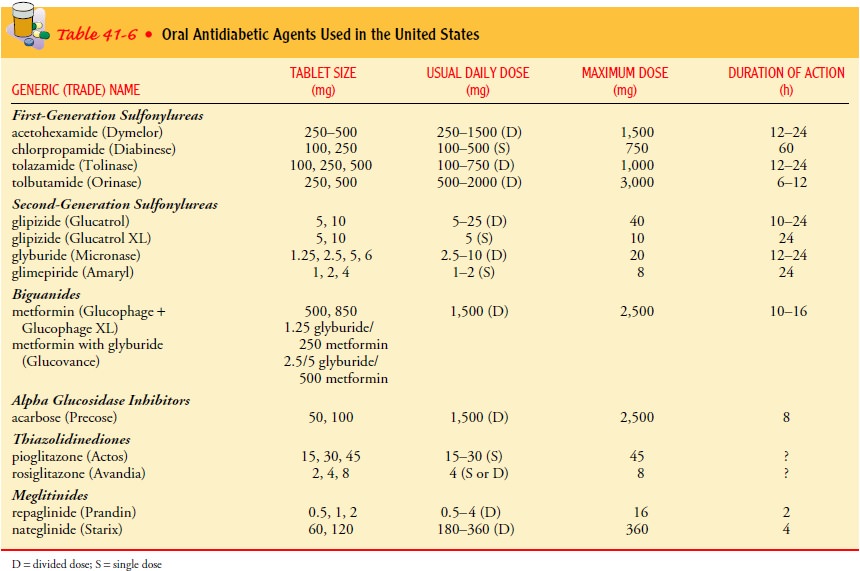
Oral Antidiabetic Agents
Oral
antidiabetic agents may be effective for patients who have type 2 diabetes that
cannot be treated by diet and exercise alone; however, they cannot be used
during pregnancy. In the United States, oral antidiabetic agents include the
sulfonylureas, biguanides, alpha glucosidase inhibitors, thiazolidinediones,
and meglitinides (Table 41-6). Sulfonylureas and meglitinides are considered
in-sulin secretagogues because their action increases the secretion of insulin
by the pancreatic beta cells.
SULFONYLUREAS
The sulfonylureas exert their primary
action by directly stim-ulating the pancreas to secrete insulin. Therefore, a
functioning pancreas is necessary for these agents to be effective, and they
cannot be used in patients with type 1 diabetes. These agents improve insulin
action at the cellular level and may also directly decrease glucose production
by the liver. The sulfonylureas can be divided into first- and
second-generation categories (see Table 41-6).
The
most common side effects of these medications are GI symptoms and dermatologic
reactions. Hypoglycemia may occur when an excessive dose of a sulfonylurea is
used or when the patient omits or delays meals, reduces food intake, or
in-creases activity. Because of the prolonged hypoglycemic effects of these
agents (especially chlorpropamide), some patients need to be hospitalized for
treatment of oral agent-induced hypo-glycemia. Another side effect of
chlorpropamide is a disulfiram (Antabuse) type of reaction when alcohol is
ingested (see section on alcohol consumption for more information). Some
medica-tions may directly interact with sulfonylureas, potentiating their
hypoglycemic effects (eg, sulfonamides, chloramphenicol, clofi-brate,
phenylbutazone, and bishydroxycoumarin). In addition, certain medications may
independently affect blood glucose lev-els, thereby indirectly interfering with
these agents. Medications that may increase glucose levels include
potassium-losing di-uretics, corticosteroids, estrogen compounds, and
diphenylhy-dantoin (Dilantin). Medications that may cause hypoglycemia include
salicylates, propranolol, monoamine oxidase inhibitors, and pentamidine.
Second-generation
sulfonylureas have the advantage of a shorter half-life and excretion by both
the kidney and the liver. This makes these medications safer to use in the
elderly, in whom accumulation of the medication can cause recurring
hypoglycemia.
BIGUANIDES
The biguanides are other kinds of oral antidiabetic agents. Metformin (Glucophage) produces its antidiabetic effects by fa-cilitating insulin’s action on peripheral receptor sites. Therefore, it can be used only in the presence of insulin. Biguanides have no effect on pancreatic beta cells. Biguanides used with a sulfonyl-urea may enhance the glucose-lowering effect more than either medication used alone. Lactic acidosis is a potential and serious complication of biguanide therapy; the patient must be monitored closely when therapy is initiated or when dosage changes.
Medications
that may interact with biguanides include anticoagulants, corticosteroids,
diuretics, and oral contraceptives. Metformin is contraindicated in patients
with renal impairment (serum creati-nine level more than 1.4) or those at risk
for renal dysfunction (eg, those with acute myocardial infarction). Renal
function studies should be performed periodically to ensure that function is
not impaired. Metformin should not be administered for 2 days be-fore any
diagnostic testing that may require use of a contrast agent. These situations
increase the risk for lactic acidosis.
An
extended-release form and a combination form (Gluco-vance) combines metformin
with a sulfonylurea, such as glyburide. The combination provides two mechanisms
of action and im-proved patient compliance. Hypoglycemia is a risk.
ALPHA GLUCOSIDASE INHIBITORS
Acarbose
(Precose) and miglitol (Glyset) are oral alpha
glucosi-dase inhibitors used in type 2 diabetes management. They workby
delaying the absorption of glucose in the intestinal system, re-sulting in a
lower postprandial blood glucose level. As a conse-quence of plasma glucose
reduction, hemoglobin A1C levels drop. In contrast to the sulfonylureas,
acarbose and miglitol do not enhance insulin secretion. They can be used alone
with dietary treatment as monotherapy or in combination with sulfonylureas,
thiazolidinediones, or meglitinides. When these medications are used in
combination with sulfonylureas or meglitinides, hypo-glycemia may occur. The
patient must be advised that if hypo-glycemia occurs, sucrose absorption will
be blocked and treatment for hypoglycemia should be in the form of glucose,
such as glu-cose tablets. The advantage of oral alpha glucosidase inhibitors is
that they are not systemically absorbed and are safe to use. Their side effects
are diarrhea and flatulence. These effects may be min-imized by starting at a
very low dose and increasing the dose grad-ually. Because acarbose and miglitol
affect food absorption, they must be taken immediately before a meal, making
therapeutic adherence a potential problem.
THIAZOLIDINEDIONES
Rosiglitizone
(Avandia) and pioglitozone (Actos) are oral diabetes medications categorized as
thiazolidinediones. They are
indi-cated for patients with type 2 diabetes who take insulin injections and
whose blood glucose control is inadequate (hemoglobin A1C level greater than
8.5%). They have also been approved as first-line agents to treat type 2
diabetes, in combination with diet. Thi-azolidinediones enhance insulin action
at the receptor site without increasing insulin secretion from the beta cells
of the pancreas. These medications may affect liver function; therefore, liver
func-tion studies must be performed at baseline and at frequent inter-vals
(monthly for the first 12 months of treatment, and quarterly thereafter). Women
should be informed that thiazolidinediones can cause resumption of ovulation in
perimenopausal anovulatory women, making pregnancy a possibility.
MEGLITINIDES
Repaglinide
(Prandin), an oral glucose-lowering agent of the class of oral agents called
meglitinides, lowers the blood glucose level by stimulating insulin release
from the pancreatic beta cells. Its effectiveness depends on the presence of
functioning beta cells. Therefore, repaglinide is contraindicated in patients
with type 1 diabetes. Repaglinide has a fast action and a short duration. It
should be taken before each meal to stimulate the release of in-sulin in
response to that meal. It is also indicated for use in com-bination with
metformin in patients whose hyperglycemia cannot be controlled by exercise,
diet, and either metformin or repaglinide alone. The principal side effect of
repaglinide is hypo-glycemia; however, this side effect is less severe and
frequent than for a sulfonylurea because repaglinide has a short half-life
(approximately 1 hour). Patients must be taught the signs and symptoms of
hypoglycemia and should understand that the medication should not be taken
unless the patient eats a meal. Repaglinide is supplied in 0.5-, 1-, and 2-mg
tablets.
Naglitinide
(Starlix), another meglitinide, has a very rapid onset and short duration. It
should be taken with meals and not taken if the meal is skipped. Hypoglycemia
risk is low if taken correctly.
General Considerations for Oral Agents
Patients
need to understand that oral agents are prescribed as an ad-dition to (not as a
substitute for) other treatment modalities, such as diet and exercise. Use of
oral antidiabetic medications may need to be halted temporarily and insulin
prescribed if hyperglycemia develops that is attributable to infection, trauma,
or surgery.
In
time, oral antidiabetic agents may no longer be effective in controlling the
patient’s diabetes. In such cases, the patient is treated with insulin.
Approximately half of all patients who ini-tially use oral antidiabetic agents
eventually require insulin. This is referred to as a secondary failure. Primary
failure occurs when the blood glucose level remains high a month after initial
med-ication use.
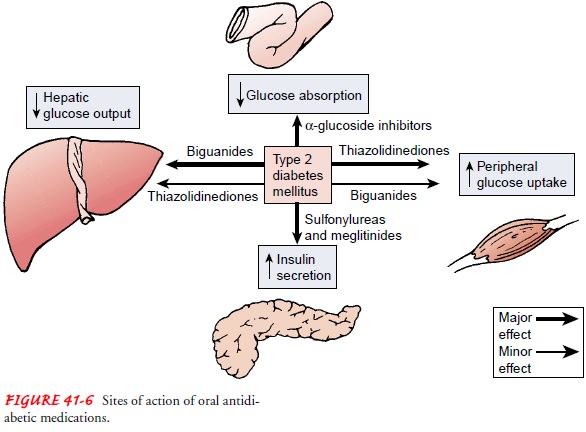
Because
the mechanisms of action vary (Fig. 41-6), the ef-fect may be enhanced using
multidose, multiple medications (Inzucchi et al., 1998). Use of multiple
medications with dif-ferent mechanisms of action is very common today (Quinn,
2001b). Using a combination of oral agents with insulin has been proposed as a
treatment for some patients with type 2 diabetes. However, the effectiveness of
this approach has not yet been demonstrated.
Related Topics