Chapter: Medical Surgical Nursing: Assessment and Management of Patients With Diabetes Mellitus
Diabetic Ketoacidosis - Acute Complications of Diabetes
DIABETIC
KETOACIDOSIS
DKA is
caused by an absence or markedly inadequate amount of insulin. This deficit in
available insulin results in disorders in the metabolism of carbohydrate,
protein, and fat. The three main clinical features of DKA are:
•
Hyperglycemia
•
Dehydration and electrolyte loss
•
Acidosis
Pathophysiology
Without
insulin, the amount of glucose entering the cells is re-duced and the liver
increases glucose production. Both factors lead to hyperglycemia. In an attempt
to rid the body of the excess glucose, the kidneys excrete the glucose along
with water and electrolytes (eg, sodium and potassium). This osmotic diuresis,
which is characterized by excessive urination (polyuria), leads to dehydration
and marked electrolyte loss. Patients with severe DKA may lose up to 6.5 liters
of water and up to 400 to 500 mEq each of sodium, potassium, and chloride over
a 24-hour period.
Another
effect of insulin deficiency or deficit is the breakdown of fat (lipolysis)
into free fatty acids and glycerol. The free fatty acids are converted into
ketone bodies by the liver. In DKA there is excessive production of ketone
bodies because of the lack of in-sulin that would normally prevent this from
occurring. Ketone bodies are acids; their accumulation in the circulation leads
to metabolic acidosis.
Three
main causes of DKA are decreased or missed dose of in-sulin, illness or
infection, and undiagnosed and untreated dia-betes (DKA may be the initial
manifestation of diabetes). An insulin deficit may result from an insufficient
dosage of insulin prescribed or from insufficient insulin being administered by
the patient. Errors in insulin dosage may be made by patients who are ill and
who assume that if they are eating less or if they are vomiting, they must
decrease their insulin doses. (Because illness, especially infections, may
cause increased blood glucose levels, pa-tients do not need to decrease their
insulin doses to compensate for decreased food intake when ill and may even
need to increase the insulin dose.)
Other
potential causes of decreased insulin include patient error in drawing up or
injecting insulin (especially in patients with visual impairments), intentional
skipping of insulin doses (especially in adolescents with diabetes who are
having difficulty coping with diabetes or other aspects of their lives), or
equipment problems (eg, occlusion of insulin pump tubing).
Illness
and infections are associated with insulin resistance. In response to physical
(and emotional) stressors, there is an increase in the level of “stress”
hormones—glucagon, epinephrine, nor-epinephrine, cortisol, and growth hormone.
These hormones promote glucose production by the liver and interfere with
glu-cose utilization by muscle and fat tissue, counteracting the effect of
insulin. If insulin levels are not increased during times of ill-ness and infection,
hyperglycemia may progress to DKA (Quinn, 2001c).
Clinical Manifestations
The
signs and symptoms of DKA are listed in Figure 41-8. The hyperglycemia of DKA
leads to polyuria and polydipsia (in-creased thirst). In addition, patients may
experience blurred vi-sion, weakness, and headache. Patients with marked
intravascular volume depletion may have orthostatic hypotension (drop in
sys-tolic blood pressure of 20 mm Hg or more on standing). Volume depletion may
also lead to frank hypotension with a weak, rapid pulse.
The ketosis and acidosis of DKA lead to GI symptoms such as anorexia, nausea, vomiting, and abdominal pain. The abdominal pain and physical findings on examination can be so severe that they resemble an acute abdominal disorder that requires surgery. Patients may have acetone breath (a fruity odor), which occurs with elevated ketone levels.
In addition, hyperventilation (with very
deep, but not labored, respirations) may occur. These Kuss-maul respirations
represent the body’s attempt to decrease the aci-dosis, counteracting the
effect of the ketone buildup. In addition, mental status changes in DKA vary
widely from patient to pa-tient. Patients may be alert, lethargic, or comatose,
most likely depending on the plasma osmolarity (concentration of osmoti-cally
active particles).
Assessment and Diagnostic Findings
Blood
glucose levels may vary from 300 to 800 mg/dL (16.6 to 44.4 mmol/L). Some
patients have lower glucose values, and oth-ers have values of 1,000 mg/dL
(55.5 mmol/L) or more (usually depending on the degree of dehydration). The
severity of DKA is not necessarily related to the blood glucose level. Some
patients may have severe acidosis with modestly elevated blood glucose levels,
whereas others may have no evidence of DKA despite blood glucose levels of 400
to 500 mg/dL (22.2 to 27.7 mmol/L) (Quinn, 2001c).
Evidence
of ketoacidosis is reflected in low serum bicarbonate (0 to 15 mEq/L) and low
pH (6.8 to 7.3) values. A low PCO2 level (10 to 30 mm Hg) reflects respiratory
compensation (Kussmaul respirations) for the metabolic acidosis. Accumulation
of ketone bodies (which precipitates the acidosis) is reflected in blood and
urine ketone measurements.
Sodium
and potassium levels may be low, normal, or high, depending on the amount of
water loss (dehydration). Despite the plasma concentration, there has been a
marked total body de-pletion of these (and other) electrolytes. Ultimately,
these elec-trolytes will need to be replaced.
Elevated
levels of creatinine, blood urea nitrogen (BUN), he-moglobin, and hematocrit
may also be seen with dehydration. After rehydration, continued elevation in
the serum creatinine and BUN levels will be present in the patient with
underlying renal insufficiency.
Prevention
For prevention
of DKA related to illness, patients must be taught “sick day” rules for
managing their diabetes when ill (Chart 41-9). The most important issue to
teach patients is not to eliminate insulin doses when nausea and vomiting
occur. Rather, they should take their usual insulin dose (or previously
prescribed special “sick day” doses) and then attempt to con-sume frequent
small portions of carbohydrates (including foods usually avoided, such as
juices, regular sodas, and gelatin). Drinking fluids every hour is important to
prevent dehydration. Blood glucose and urine ketones must be assessed every 3
to 4 hours.
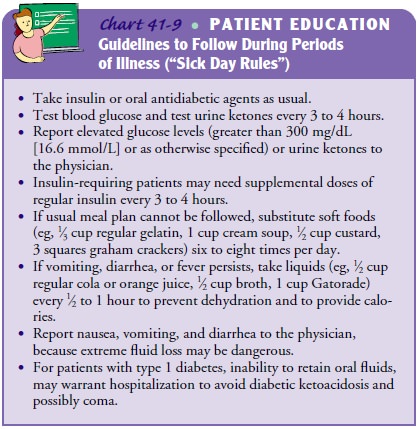
If the
patient cannot take fluids without vomiting, or if ele-vated glucose or ketone
levels persist, the physician must be con-tacted. Patients are taught to have
available foods for use on sick days. In addition, a supply of urine test
strips (for ketone testing) and blood glucose test strips should be available.
Patients must know how to contact their physician 24 hours a day.
Diabetes self-management skills (including insulin adminis-tration and blood glucose testing) should be assessed to ensure that an error in insulin administration or blood glucose testing did not occur. Psychological counseling is recommended for pa-tients and family members if an intentional alteration in insulin dosing was the cause of the DKA
Medical Management
In
addition to treating hyperglycemia, management of DKA is aimed at correcting
dehydration, electrolyte loss, and acidosis (Quinn, 2001c).
REHYDRATION
In
dehydrated patients, rehydration is important for maintaining tissue perfusion.
In addition, fluid replacement enhances the ex-cretion of excessive glucose by
the kidneys. Patients may need up to 6 to 10 liters of IV fluid to replace
fluid losses caused by polyuria, hyperventilation, diarrhea, and vomiting.
Initially,
0.9% sodium chloride (normal saline) solution is ad-ministered at a rapid rate,
usually 0.5 to 1 L per hour for 2 to 3 hours. Half-strength normal saline
(0.45%) solution (also known as hypotonic saline solution) may be used for
patients with hyper-tension or hypernatremia or those at risk for heart
failure. After the first few hours, half-normal saline solution is the fluid of
choice for continued rehydration, if the blood pressure is sta-ble and the
sodium level is not low. Moderate to high rates of infusion (200 to 500 mL per
hour) may continue for several more hours. When the blood glucose level reaches
300 mg/dL (16.6 mmol/L) or less, the IV fluid may be changed to dextrose 5% in
water (D5W) to prevent a
precipitous decline in the blood glucose level (ADA, Hyperglycemic Crisis in
Patients with Dia-betes Mellitus, 2003).
Monitoring
fluid volume status involves frequent measure-ments of vital signs (including
monitoring for orthostatic changes in blood pressure and heart rate), lung
assessment, and monitor-ing intake and output. Initial urine output will lag
behind IV fluid intake as dehydration is corrected. Plasma expanders may be
necessary to correct severe hypotension that does not respond to IV fluid
treatment. Monitoring for signs of fluid overload is es-pecially important for
older patients, those with renal impair-ment, or those at risk for heart
failure.
RESTORING ELECTROLYTES
The
major electrolyte of concern during treatment of DKA is potassium. Although the
initial plasma concentration of potas-sium may be low, normal, or even high,
there is a major loss of potassium from body stores and an intracellular to
extracellular shift of potassium. Further, the serum level of potassium drops
during the course of treatment of DKA as potassium re-enters the cells;
therefore, it must be monitored frequently. Some of the fac-tors related to
treating DKA that reduce the serum potassium concentration include:
•
Rehydration, which leads to increased plasma volume
and subsequent decreases in the concentration of serum potas-sium. Rehydration
also leads to increased urinary excretion of potassium.
•
Insulin administration, which enhances the movement
of potassium from the extracellular fluid into the cells.
Cautious
but timely potassium replacement is vital to avoid dysrhythmias that may occur
with hypokalemia. Up to 40 mEq per hour may be needed for several hours.
Because extracellular potassium levels drop during DKA treatment, potassium
must be infused even if the plasma potassium level is normal.
Frequent
(every 2 to 4 hours initially) electrocardiograms and laboratory measurements
of potassium are necessary during the first 8 hours of treatment. Potassium
replacement is withheld only if hyperkalemia is present or if the patient is
not urinating.
REVERSING ACIDOSIS
Ketone
bodies (acids) accumulate as a result of fat breakdown. The acidosis that
occurs in DKA is reversed with insulin, which inhibits fat breakdown, thereby stopping acid buildup. Insulin is usually infused
intravenously at a slow, continuous rate (eg, 5 units per hour). Hourly blood
glucose values must be mea-sured. IV fluid solutions with higher concentrations
of glucose, such as normal saline (NS) solution (eg, D5NS or D50.45NS),
are administered when blood glucose levels reach 250 to 300 mg/dL (13.8 to 16.6
mmol/L) to avoid too rapid a drop in the blood glucose level.
Various IV mixtures of regular insulin may be
used. The nurse must convert hourly rates of insulin infusion (frequently
pre-scribed as “units per hour”) to IV drip rates. For example, if 100 units of
regular insulin are mixed in 500 mL 0.9% NS, then 1 unit of insulin equals 5
mL. Thus, an initial insulin infusion rate of 5 units per hour would equal 25
mL per hour. The insulin is often infused separately from the rehydration
solutions to allow frequent changes in the rate and content of rehydration
solutions.
Insulin
must be infused continuously until subcutaneous administration of insulin
resumes. Any interruption in administration may result in the reaccumulation of
ketone bodies and worsening acidosis. Even if blood glucose levels are dropping
to normal, the insulin drip must not be stopped; rather, the rate or
concentration of the dextrose infusion should be increased. Blood glucose
levels are usually corrected before the acidosis is corrected. Thus, IV insulin
may be continued for 12 to 24 hours until the serum bicarbonate level improves
(to at least 15 to 18 mEq/L) and until the patient can eat. In general,
bicarbonate infusion to correct severe acidosis is avoided during treatment of
DKA be-cause it precipitates further, sudden (and potentially fatal) de-creases
in serum potassium levels. Continuous insulin infusion is usually sufficient for
reversing DKA.
Nursing Management
Nursing
care of the patient with DKA focuses on monitoring fluid and electrolyte status
as well as blood glucose levels; admin-istering fluids, insulin, and other
medications; and preventing other complications such as fluid overload. Urine
output is mon-itored to ensure adequate renal function before potassium is
ad-ministered to prevent hyperkalemia. The electrocardiogram is monitored for
dysrhythmias indicating abnormal potassium lev-els. Vital signs, arterial blood
gases, and other clinical findings are recorded on a flow sheet. The nurse
documents the patient’s lab-oratory values and the frequent changes in fluids
and medications that are prescribed and monitors the patient’s responses. As
DKA resolves and the potassium replacement rate is decreased, the nurse makes
sure that:
•
There are no signs of hyperkalemia on the
electrocardio-gram (tall, peaked [or tented] T waves).
•
The laboratory values of potassium are normal or
low.
•
The patient is urinating (ie, no renal shutdown).
As the
patient recovers, the nurse reassesses the factors that may have led to DKA and
teaches the patient and family about strategies to prevent its recurrence
(Quinn, 2001c). If indicated,the nurse initiates a referral for home care to
ensure the patient’s continued recovery.
Related Topics