Chapter: Medical Surgical Nursing: Assessment and Management of Patients With Hepatic Disorders
Management of Patients With Hepatitis C Virus (HCV)
HEPATITIS
C VIRUS (HCV)
A
significant proportion of cases of viral hepatitis are neither hep-atitis A,
hepatitis B, nor hepatitis D; as a result, they are classified as hepatitis C
(formerly referred to as non-A, non-B hepatitis, or NANB hepatitis). Whereas
blood transfusions and sexual contact once accounted for most cases of
hepatitis C in the United States, other parenteral means, such as sharing
contaminated needles by IV/injection drug users and unintentional needlesticks
and other injuries in health care workers, now account for a significant number
of cases. There are approximately 35,000 new cases of hepatitis C in the United
States each year. About 4 million per-sons (1.8% of the U.S. population) have
been infected with HCV, making it the most common chronic blood-borne
infec-tion nationally. A fourfold increase in the number of adults diag-nosed
with HCV is projected from 1990 to 2015. The highest prevalence of hepatitis C
is in adults 40 to 59 years of age, and in this age group its prevalance is
highest in African Americans. There are 10,000 to 12,000 deaths each year in
the United States due to hepatitis C; it has been suggested that these are
underesti-mates. HCV is the underlying cause of about one-third of cases of
hepatocellular carcinoma, and it is the most common reason for liver
transplantation (NIH Consensus Conference, 2002).
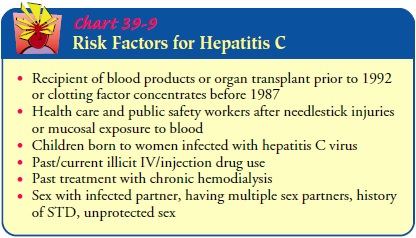
Individuals
at special risk for hepatitis C include IV/injection drug users, sexually
active people with multiple partners, patients receiving frequent transfusions
or those who require large vol-umes of blood, and health care personnel. The
incubation period is variable and may range from 15 to 160 days. The clinical
course of acute hepatitis C is similar to that of hepatitis B; symptoms are
usually mild. A chronic carrier state occurs frequently, however, and there is
an increased risk of chronic liver disease, including cirrhosis or liver cancer,
after hepatitis C. Small amounts of alco-hol taken regularly appear to
encourage progression of the dis-ease. Therefore, alcohol and medications that
may affect the liver should be avoided (Chart 39-9).
There
is no benefit from rest, diet, or vitamin supplements. Re-cent studies have
demonstrated that a combination of interferon (Intron-A) and ribavirin
(Rebetol), two antiviral agents, is effective in producing improvement in
patients with hepatitis C and in treating relapses. Some patients experience complete
remission with combination therapy, which is the treatment of choice according
to the FDA (Cheney, Chopra & Graham, 2000). He-molytic anemia, the most
frequent side effect, may be severe enough to require discontinuation of
treatment. Ribavirin must be used with caution in women of childbearing age. A
molecule (poly-ethylene glycol moiety [PEG]) added to the interferon keeps it
in the body longer without reducing its efficacy and extends the dos-ing
interval to once a week. Pegylated interferon (Pegasys) is now available (Lauer
& Walker, 2001; Sheffield et al., 2001).
Screening of blood has reduced the incidence of hepatitis as-sociated with blood transfusions, and public health programs are helping to reduce the number of cases associated with shared needles in illicit drug use.
Related Topics