Chapter: Medical Surgical Nursing: Assessment and Management of Patients With Hepatic Disorders
Esophageal Varices - Hepatic Dysfunction
ESOPHAGEAL
VARICES
Bleeding
or hemorrhage from esophageal varices occurs in approx-imately one third of
patients with cirrhosis and varices. The mor-tality rate resulting from the
first bleeding episode is 45% to 50%; it is one of the major causes of death in
patients with cirrhosis (Pomier-Layrargues, Villeneuve, Deschenes et al.,
2001). The mor-tality rate increases with each subsequent bleeding episode.
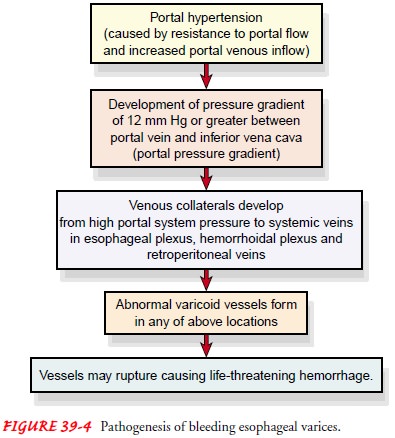
Pathophysiology
Esophageal
varices are dilated, tortuous veins usually found in the submucosa of the lower
esophagus, but they may develop higher in the esophagus or extend into the
stomach. This condition nearly always is caused by portal hypertension, which
in turn is due to ob-struction of the portal venous circulation within the
damaged liver.
Because
of increased obstruction of the portal vein, venous blood from the intestinal
tract and spleen seeks an outlet through collateral circulation (new pathways
of return to the right atrium). The effect is increased pressure, particularly
in the vessels in the submucosal layer of the lower esophagus and upper part of
the stomach. These collateral vessels are not very elastic but rather are
tortuous and fragile and bleed easily (Fig. 39-6). Less common causes of
varices are abnormalities of the circulation in the splenic vein or superior
vena cava and hepatic venothrombosis.
Bleeding
esophageal varices are life-threatening and can result in hemorrhagic shock,
producing decreased cerebral, hepatic, and renal perfusion. In turn, there is
an increased nitrogen load from bleeding into the GI tract and an increased
serum ammonia level, increasing the risk for encephalopathy. Usually the
dilated veins cause no symptoms unless the portal pressure increases sharply
and the mucosa or supporting structures become thin. Then massive hemorrhage
takes place.
Factors
that contribute to hemorrhage are muscular exertion from lifting heavy objects;
straining at stool; sneezing, coughing, or vomiting; esophagitis; irritation of vessels
by poorly chewed foods or irritating fluids; or reflux of stomach contents
(especially alcohol). Salicylates and any medication that erodes the esophageal
mucosa or interferes with cell replication also may contribute to bleeding.
Clinical Manifestations
The patient with bleeding esophageal varices may present with hematemesis, melena, or general deterioration in mental or phys-ical status and often has a history of alcohol abuse. Signs and symptoms of shock (cool clammy skin, hypotension, tachycardia) may be present.
Assessment and Diagnostic Findings
Endoscopy
is used to identify the bleeding site, along with barium swallow,
ultrasonography, CT, and angiography.
ENDOSCOPY
Immediate
endoscopy is indicated to identify the cause and the site of bleeding; at least
30% of patients suspected of bleeding from esophageal varices bleed from other
sources (gastritis, ulcers). Nursing support can be effective in relieving
anxiety during this often-stressful experience. Careful monitoring can detect
early signs of cardiac dysrhythmias, perforation, and hemorrhage.
After
the examination, fluids are not given until the gag reflex returns. Lozenges
and gargles may be used to relieve throat dis-comfort if the patient’s physical
condition and mental status per-mit. If the patient is actively bleeding, oral
intake will not be permitted and the patient will be prepared for further
diagnostic and therapeutic procedures.
PORTAL HYPERTENSION MEASUREMENTS
Portal
hypertension may be suspected if dilated abdominal veins and hemorrhoids are
detected. A palpable enlarged spleen (splenomegaly) and ascites may also be
present. Portal venous pressure can be measured directly or indirectly.
Indirect mea-surement of the hepatic vein pressure gradient is the most com-mon
procedure; it requires insertion of a fluid-filled balloon catheter into the
antecubital or femoral vein. The catheter is ad-vanced under fluoroscopy to a
hepatic vein. A “wedged” pressure (similar to pulmonary artery wedge pressure)
is obtained by oc-cluding the blood flow in the blood vessel; pressure in the
unoc-cluded vessel is also measured. Although the values obtained may
underestimate portal pressure, this measurement may be ob-tained several times
to evaluate the results of therapy.
Direct
measurement of portal vein pressure can be obtained by several methods. During
laparotomy, a needle may be intro-duced into the spleen; a manometer reading of
more than 20 mL saline is abnormal. Another direct measurement requires
inser-tion of a catheter into the portal vein or one of its branches.
En-doscopic measurement of pressure within varices is used only in conjunction
with endoscopic sclerotherapy.
LABORATORY TESTS
Laboratory
tests may include various liver function tests, such as serum aminotransferase,
bilirubin, alkaline phosphatase, and serum proteins. Splenoportography, which
involves serial or segmental x-rays, is used to detect extensive collateral
circulation in esoph-ageal vessels, which would indicate varices. Other tests
are hepato-portography and celiac angiography. These are usually performed in
the operating room or radiology department.
Medical Management
Bleeding
from esophageal varices can quickly lead to hemor-rhagic shock and is an
emergency. This patient is critically ill, requiring aggressive medical care
and expert nursing care, and is usually transferred to the intensive care unit
for close moni-toring and management.
The
extent of bleeding is evaluated and vital signs are monitored continuously when
hematemesis and melena are present. Signs of potential hypovolemia are noted,
such as cold clammy skin, tachy-cardia, a drop in blood pressure, decreased
urine output, restless-ness, and weak peripheral pulses. Blood volume is
monitored by a central venous pressure or arterial catheter. Oxygen is administered
to prevent hypoxia and to maintain adequate blood oxygenation.
Because
patients with bleeding esophageal varices have intra-vascular volume depletion
and are subject to electrolyte imbal-ance, intravenous fluids with electrolytes
and volume expanders are provided to restore fluid volume and replace
electrolytes. Trans-fusion of blood components also may be required. An
indwelling urinary catheter is usually inserted to permit frequent monitoring
of urine output.
A
variety of pharmacologic, endoscopic, and surgical approaches are used to treat
bleeding esophageal varices, but none is ideal and most are associated with
considerable risk to the patient. Non-surgical treatment of bleeding esophageal
varices is preferable be-cause of the high mortality rate of emergency surgery
for control of bleeding esophageal varices and because of the poor physical
condition of the patient with severe liver dysfunction.
PHARMACOLOGIC THERAPY
In an actively bleeding patient, medications
are administered ini-tially because they can be obtained and administered
quickly; other therapies take longer to initiate. Vasopressin (Pitressin) may
be the initial mode of therapy because it produces constriction of the
splanchnic arterial bed and a resulting decrease in portal pressure. It may be
administered intravenously or by intra-arterial infusion (Menon & Kamath,
2000). Either method requires close moni-toring by the nurse. Vital signs and
the presence or absence of blood in the gastric aspirate indicate the
effectiveness of vasopressin. Monitoring of fluid intake and output and
electrolyte levels is nec-essary because hyponatremia may occur and vasopressin
may have an antidiuretic effect. Coronary artery disease is a contraindication
to the use of vasopressin, because coronary vasoconstriction is a side effect
that may precipitate myocardial infarction.
The
combination of vasopressin and nitroglycerin (adminis-tered by the intravenous,
sublingual, or transdermal route) has been effective in reducing or preventing
the side effects (constriction of coronary vessels and angina) caused by
vasopressin alone.
Somatostatin
and octreotide (Sandostatin) have been reported to be more effective than
vasopressin in decreasing bleeding from esophageal varices without the
vasoconstrictive effects of vaso-pressin. These medications cause selective
splanchnic vasoconstric-tion. Propranolol (Inderal) and nadolol (Corgard),
beta-blocking agents that decrease portal pressure, have been shown to prevent
bleeding from esophageal varices in some patients; however, it is recommended
that they be used only in combination with other treatment modalities such as
sclerotherapy, variceal banding, or balloon tamponade. Nitrates such as
isosorbide (Isordil) lower por-tal pressure by venodilation and decreased cardiac
output. Further studies of these and other medications are necessary to
evaluate their use in the treatment and prevention of bleeding episodes.
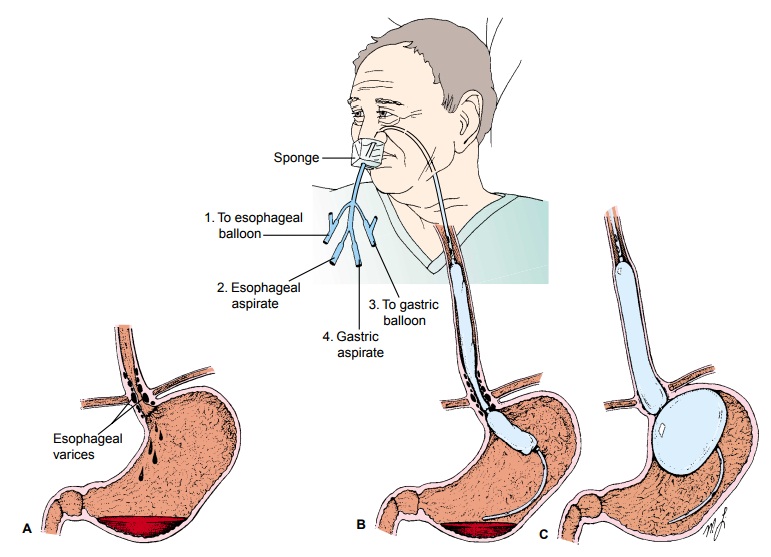
BALLOON TAMPONADE
To
control hemorrhage in certain patients, balloon
tamponade may be used. In this procedure, pressure is exerted on the cardia
(upper orifice of the stomach) and against the bleeding varices by a
double-balloon tamponade (Sengstaken-Blakemore tube) (Fig. 39-7). The tube has
four openings, each with a specific pur-pose: gastric aspiration, esophageal
aspiration, inflation of the gastric balloon, and inflation of the esophageal
balloon.
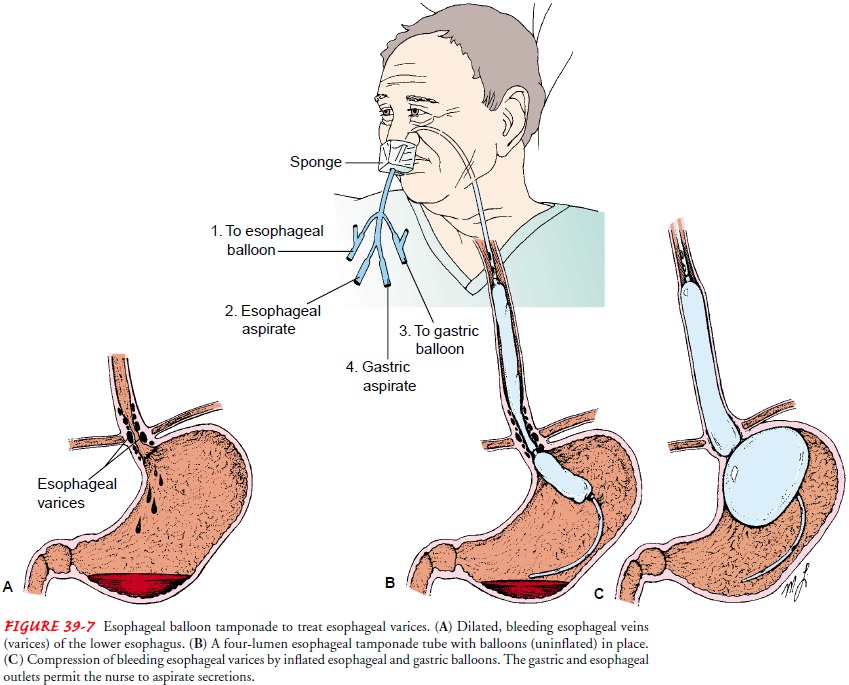
The balloon in the stomach is inflated with 100 to 200 mL of air. An x-ray confirms proper positioning of the gastric balloon. Then the tube is pulled gently to exert a force against the gastric cardia.
Traction may be applied with weights or by attachment to a football helmet.
Irrigation of the tubing is performed to de-tect bleeding; if returns are
clear, the esophageal balloon is not in-flated. If bleeding continues, the
esophageal balloon is inflated. The desired pressure in the esophageal and
gastric balloons is 25 to 40 mm Hg, as measured by the manometer. There is a
possi-bility of injury or rupture of the esophagus with inflation of the
esophageal balloon, so constant nursing surveillance is necessary.
Gastric
suction is provided by connecting the gastric catheter outlet to suction. The
tubing is irrigated hourly, and drainage will indicate whether bleeding has
been controlled. Room-temperature lavage or irrigation may be used in the
gastric balloon. The pressure within the esophageal balloon is measured and
recorded every 2 to 4 hours via the manometer to detect underinflation or
over-inflation with potential for esophageal injury. When it appears that
bleeding has stopped, the balloons are carefully and sequentially deflated. The
esophageal balloon is deflated first and the patient is monitored for recurrent
bleeding. After several hours without bleeding, the gastric balloon may be
deflated safely. If there is still no bleeding, the tamponade tube is removed.
The therapy is used for as short a time as possible to control bleeding while
emergency treatment is completed and definitive therapies are instituted (no
longer than 24 hours).
Although
balloon tamponade has been fairly successful, there are some inherent dangers.
Displacement of the tube and the in-flated balloon into the oropharynx can
cause life-threatening ob-struction of the airway and asphyxiation. This may
occur if a patient pulls on the tube because of confusion or discomfort. It may
also result from rupture of the gastric balloon, allowing the esophageal
balloon to move into the oropharynx. Sudden rupture of the balloon causes
airway obstruction and aspiration of gastric contents into the lungs. The tube
is tested before insertion to minimize this risk. Aspiration of blood and
secretions into the lungs is frequently associated with balloon tamponade,
especially in the stuporous or comatose patient. Endotracheal intubation before
insertion of the tube protects the airway and minimizes the risk of aspiration.
Ulceration and necrosis of the nose, the mu-cosa of the stomach, or the
esophagus may occur if the tube is left in place or inflated too long or at too
high a pressure.
These
potential complications necessitate intensive and expert care. A confused or
restless patient with this tube in place and bal-loons inflated requires close
monitoring to prevent its displacement. Nursing measures include frequent mouth
and nasal care. For se-cretions that accumulate in the mouth, tissues should be
within easy reach of the patient. Oral suction may be necessary to re-move oral
secretions. Because of the many potential complications, balloon tamponade
tubes are used only as a temporary measure.
The
patient with esophageal hemorrhage is usually extremely anxious and frightened.
Knowing that the nurse is nearby and will respond immediately can help
alleviate some of this anxiety. Tube insertion is uncomfortable and never
pleasant. Explana-tions during the procedure and while the tube is in place may
be reassuring to the patient.
Although
the use of balloon tamponade stops the bleeding in 90% of patients, bleeding
recurs in 60% to 70%, necessitating other treatment modalities (eg,
sclerotherapy or banding) (Menon Kamath, 2000). Once the balloons are deflated
or the tube is re-moved, the patient must be assessed frequently because of the
high risk for recurrent bleeding.
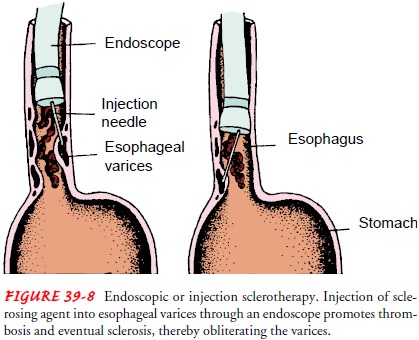
ENDOSCOPIC SCLEROTHERAPY
In
endoscopic sclerotherapy (Fig. 39-8)
(also referred to as injec-tion sclerotherapy), a sclerosing agent is injected
through a fiberop-tic endoscope into the bleeding esophageal varices to promote
thrombosis and eventual sclerosis. The procedure has been used suc-cessfully to
treat acute GI hemorrhage (Menon & Kamath, 2000; O’Grady et al., 2000).
Endoscopic variceal sclerotherapy has been used in the primary prophylaxis of
variceal bleeding, but the results are poorer than those of pharmacotherapy
(Sarin, Lamba, Kumar et al., 1999).
After
treatment, the patient must be observed for bleeding, per-foration of the
esophagus, aspiration pneumonia, and esophageal stricture. Antacids may be
administered after the procedure to counteract the effects of peptic reflux.
ESOPHAGEAL BANDING THERAPY (VARICEAL BAND LIGATION)
In variceal banding (Fig. 39-9), a
modified endoscope loaded with an elastic rubber band is passed through an
overtube directly onto the varix (or varices) to be banded. After suctioning
the bleeding varix into the tip of the endoscope, the rubber band is slipped
over the tissue, causing necrosis, ulceration, and eventual sloughing of the
varix.
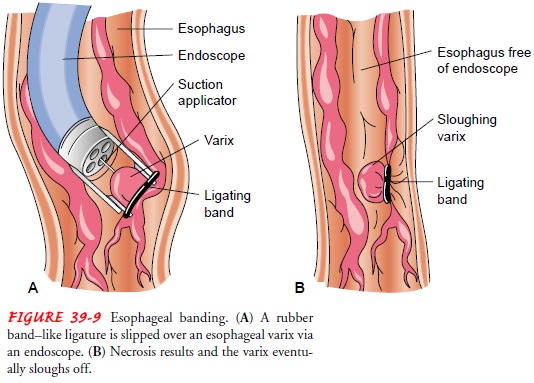
Variceal
banding is comparable to endoscopic sclerotherapy in the effective control of
bleeding. Compared with sclerotherapy, variceal banding also significantly
reduces rebleeding rates, mortal-ity, procedure-related complications, and the
number of sessions needed to eradicate varices. Complications include
superficial ul-ceration and dysphagia, transient chest discomfort, and rarely
esophageal strictures (Menon & Kamath, 2000). Recently, en-doscopic
variceal band ligation has been shown to be safe and more effective than
propanolol for preventing a first bleeding episode (Sarin et al., 1999).
TRANSJUGULAR INTRAHEPATIC PORTOSYSTEMIC SHUNTING
Transjugular
intrahepatic portosystemic shunting (TIPS) is a method of treating esophageal
varices in which a cannula is threaded into the portal vein by the transjugular
route. An expandable stent is inserted and serves as an intrahepatic shunt
between the portal circulation and the hepatic vein (Fig. 39-10), reducing
portal hypertension.
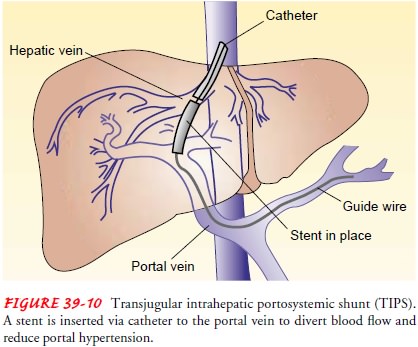
Creation
of a TIPS is indicated for the treatment of recurrent variceal bleeding
refractory to pharmacologic or endoscopic ther-apy. It has also been indicated
for the control of refractory ascites. This technique is also used as a bridge
to liver transplantation. Complications may include bleeding, sepsis, heart
failure, organ perforation, shunt thrombosis, and progressive liver failure
(Pomier-Layrargues et al., 2001).
SURGICAL MANAGEMENT
Several surgical procedures have been
developed to treat esophageal varices and to minimize rebleeding, but they are
often accompa-nied by significant risk. Procedures that may be used for
esophageal varices are direct surgical ligation of varices; splenorenal,
meso-caval, and portacaval venous shunts to relieve portal pressure; and
esophageal transection with devascularization. Use of these pro-cedures is
controversial, and studies regarding their effectiveness and outcomes are
ongoing (Bacon & Di Bisceglie, 2000).
Surgical Bypass Procedures.
Surgical
decompression of the por-tal circulation can prevent variceal bleeding if the
shunt remains patent (Bacon & Di Bisceglie, 2000). One of the various
surgical shunting procedures (Fig. 39-11) is the distal splenorenal shunt made
between the splenic vein and the left renal vein after splenectomy. A mesocaval
shunt is created by anastomosing the superior mesenteric vein to the proximal
end of the vena cava or to the side of the vena cava using grafting material.
The goal of distal splenorenal and mesocaval shunts is to drain only a portion
of venous blood from the portal bed to decrease portal pressure; thus, they are
considered selective shunts. The liver continues to receive some portal flow,
and the incidence of encephalopathy may be reduced. Portacaval shunts divert
all portal flow to the vena cava via end-to-side or side-to-side approaches, so
they are considered nonselective shunts.
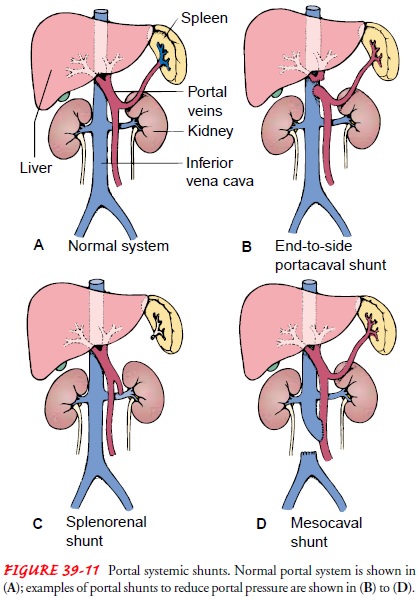
These procedures are extensive and are not always successful be-cause of secondary thrombosis in the veins used for the shunt as well as complications (eg, encephalopathy, accelerated liver failure). The efficacy of these procedures has been studied extensively. The most recent studies have found that all shunts are equally effective in preventing recurrent variceal bleeding but may cause further im-pairment of liver function and encephalopathy. Partial portacaval shunts with interposition grafts are as effective as other shunts but are associated with a lower rate of encephalopathy (de Franchis, 2000; Krige & Beckingham, 2001; Orozco & Mercado, 2000).
The severity of the disease (by Child’s classification) and the potential
for future liver transplantation guide the physician’s choice of intervention.
If the cause of portal hypertension is the rare Budd-Chiari syndrome or other venous obstructive disease,
aportacaval or a mesoatrial shunt may be performed (see Fig. 39-11). The
mesoatrial shunt is required when the infrahepatic vena cava is thrombosed and
must be bypassed.
Devascularization and Transection.
Devascularization and staple-gun transection procedures to separate the bleeding site from the high-pressure portal system have been used in the emergency management of variceal bleeding. The lower end of the esopha-gus is reached through a small gastrostomy incision; a staple gun permits anastomosis of the transected ends of the esophagus. Re-bleeding is a risk, and the outcomes of these procedures vary among patient populations.
Nursing Management
Overall
nursing assessment includes monitoring the patient’s phys-ical condition and
evaluating emotional responses and cognitive sta-tus. The nurse monitors and
records vital signs and assesses the patient’s nutritional and neurologic
status. This assessment will as-sist in identifying hepatic encephalopathy
resulting from the break-down of blood in the GI tract and a rising serum
ammonia level. Manifestations range from drowsiness to encephalopathy and coma.
Complete rest of the esophagus may be indicated with bleed-ing, so parenteral nutrition is initiated. Gastric suction usually is initiated
to keep the stomach as empty as possible and to prevent straining and vomiting.
The patient often complains of severe thirst, which may be relieved by frequent
oral hygiene and moist sponges to the lips. The nurse closely monitors the
blood pres-sure. Vitamin K therapy and multiple blood transfusions often are
indicated because of blood loss. A quiet environment and calm reassurance may
help to relieve the patient’s anxiety and reduce agitation.
Bleeding
anywhere in the body is anxiety-provoking, resulting in a crisis for the
patient and family. If the patient has been a heavy user of alcohol, delirium
secondary to alcohol withdrawal can complicate the situation. The nurse
provides support and ex-planations regarding medical and nursing interventions.
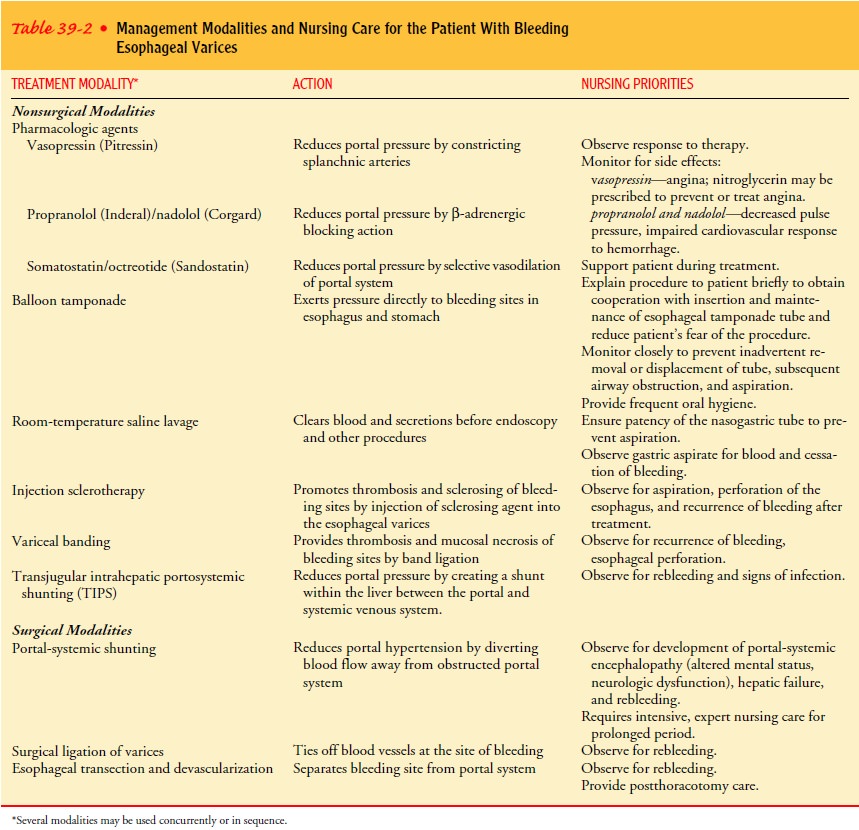
Moni-toring the patient closely will help in detecting and managing complications.Management
modalities and nursing care of the patient with bleeding esophageal varices are
summarized in Table 39-2.
Related Topics