Chapter: Medical Surgical Nursing: Assessment and Management of Patients With Eye and Vision Disorders
Glaucoma
Glaucoma
Glaucoma is a group of ocular conditions
characterized by optic nerve damage. The optic nerve damage is related to the
IOP caused by congestion of aqueous humor in the eye. There is a range of
pressures that have been considered “normal” but that may be associated with
vision loss in some patients. Glaucoma is one of the leading causes of
irreversible blindness in the world and is the leading cause of blindness among
adults in the United States. It is estimated that at least 2 million Americans
have glau-coma and that 5 to 10 million more are at risk (Margolis et al.,
2002). Glaucoma is more prevalent among people older than 40 years of age, and
the incidence increases with age. It is also more prevalent among men than
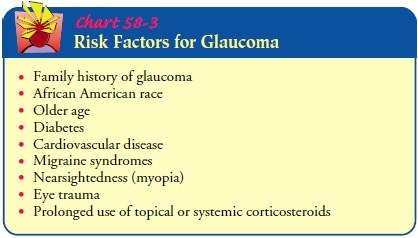
women and in the African American and Asian populations (Chart 58-3). There is
no cure for glaucoma, but research continues.
Aqueous Humor and Intraocular Pressure
Aqueous humor flows between the iris and the lens,
nourishing the cornea and lens. Most (90%) of the fluid then flows out of the
anterior chamber, draining through the spongy trabecular meshwork into the
canal of Schlemm and the episcleral veins (Fig. 58-7). About 10% of the aqueous
fluid exits through the cil-iary body into the suprachoroidal space and then
drains into the venous circulation of the ciliary body, choroid, and sclera.
Unim-peded outflow of aqueous fluid depends on an intact drainage sys-tem and
an open angle (about 45 degrees) between the iris and the cornea. A narrower
angle places the iris closer to the trabecu-lar meshwork, diminishing the angle.
The amount of aqueous humor produced tends to decrease with age, in systemic
diseases such as diabetes, and in ocular inflammatory conditions.
IOP is determined by the rate of aqueous
production, the re-sistance encountered by the aqueous humor as it flows out of
the passages, and the venous pressure of the episcleral veins that drain into
the anterior ciliary vein. When aqueous fluid production and drainage are in
balance, the IOP is between 10 and 21 mm Hg. When aqueous fluid is inhibited
from flowing out, pressure builds up within the eye. Fluctuations in IOP occur
with time of day, exertion, diet, and medications. It tends to increase with
blinking, tight lid squeezing, and upward gazing. Systemic con-ditions such as
hypertension and intraocular conditions such as uveitis and retinal detachment
have been associated with elevated IOP. Exposure to cold weather, alcohol, a
fat-free diet, heroin, and marijuana have been found to lower IOP.
Pathophysiology
There are two accepted theories regarding how
increased IOP damages the optic nerve in glaucoma. The direct mechanical
the-ory suggests that high IOP damages the retinal layer as it passes through
the optic nerve head. The indirect ischemic theory sug-gests that high IOP
compresses the microcirculation in the optic nerve head, resulting in cell
injury and death. Some glaucomas appear as exclusively mechanical, and some are
exclusively is-chemic types. Typically, most cases are a combination of both.
Regardless of the cause of damage, glaucomatous
changes typ-ically evolve through clearly discernible stages:
Initiating events: precipitating factors include illness, emo-tional stress, congenital narrow angles, long-term use of corticosteroids, and mydriatics (ie, medications causing pupillary dilation). These events lead to the second stage.
Structural
alterations in the aqueous outflow system: tissueand cellular changes caused by factors that affect
aqueous humor dynamics lead to structural alterations and to the third stage.
Functional
alterations: conditions such as
increased IOP orimpaired blood flow create functional changes that lead to the
fourth stage.
Optic
nerve damage: atrophy of the optic
nerve is charac-terized by loss of nerve fibers and blood supply, and this fourth
stage inevitably progresses to the fifth stage.
Visual
loss: progressive loss of
vision is characterized byvisual field defects.
Classification of Glaucoma
There are several types of glaucoma. Whether
glaucoma is known as open-angle or angle-closure glaucoma depends on which
mech-anisms cause impaired aqueous outflow. Glaucoma can be primary or
secondary, depending on whether associated factors con-tribute to the rise in
IOP.
Although glaucoma classification is changing as
knowledge in-creases, current clinical forms of glaucoma are open-angle
glau-comas, angle-closure glaucomas (also called pupillary block), congenital
glaucomas, and glaucomas associated with other con-ditions, such as
developmental anomalies, corticosteroid use, and other ocular conditions. The
two common clinical forms of glau-coma encountered in adults are open-angle and
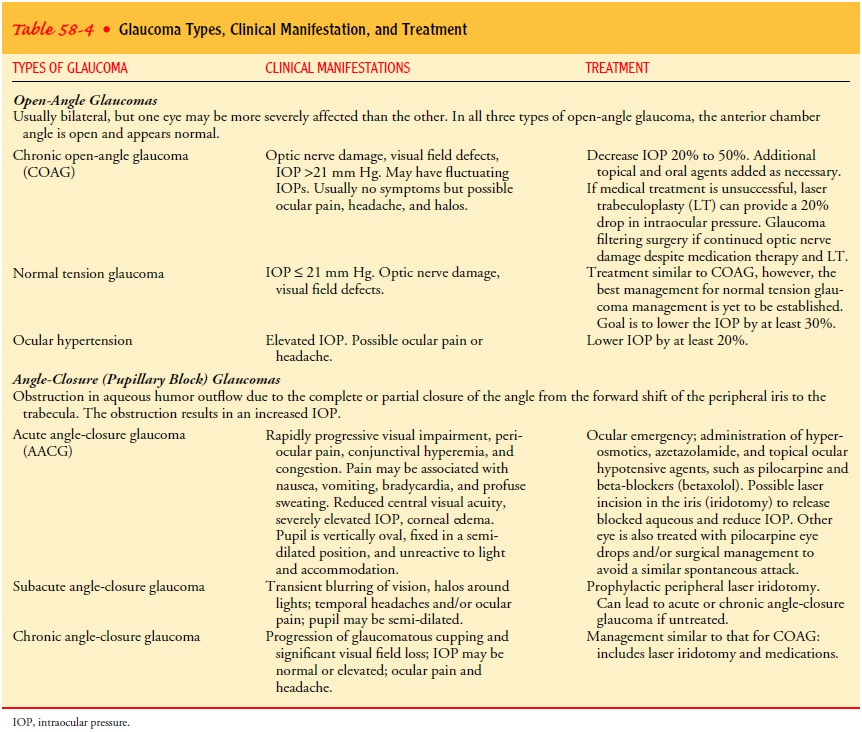
angle-closure glaucoma. Table 58-4 explains the general characteristics of the
different types of open-angle and angle-closure glaucomas.
Clinical Manifestations
Glaucoma is often called the silent thief of sight because most pa-tients are unaware that they have the disease until they have ex-perienced visual changes and vision loss. The patient may not seek health care until he or she experiences blurred vision or “halos” around lights, difficulty focusing, difficulty adjusting eyes in low lighting, loss of peripheral vision, aching or discomfort around the eyes, and headache.
Assessment and Diagnostic Findings
The
purpose of a glaucoma workup is to establish the diagnostic category, assess
the optic nerve damage, and formulate a treat-ment plan. The patient’s ocular
and medical history must be de-tailed to investigate the history of
predisposing factors. There are four major types of examinations used in
glaucoma evaluation, diagnosis, and management: tonometry to measure the IOP,
ophthalmoscopy to inspect the optic nerve, gonioscopy to exam-ine the
filtration angle of the anterior chamber, and perimetry to assess the visual
fields.
The
changes in the optic nerve significant for the diagnosis of glaucoma are pallor
and cupping of the optic nerve disc. The pal-lor of the optic nerve is caused
by a lack of blood supply that re-sults from cellular destruction. Cupping is
characterized by exaggerated bending of the blood vessels as they cross the
optic disc, resulting in an enlarged optic cup that appears more basin-like
compared with a normal cup. The progression of cupping in glaucoma is caused by
the gradual loss of retinal nerve fibers ac-companied by the loss of blood
supply, resulting in increased pal-lor of the optic disc.
As the optic nerve damage increases, visual perception in the area is lost. The localized areas of visual loss (ie, scotomas) represent loss of retinal sensitivity and are measured and mapped by perime-try. The results are mapped on a graph. In patients with glaucoma, the graph has a distinct pattern that is different from other ocular diseases and is useful in establishing the diagnosis. Figure 58-8 shows the progression of visual field defects caused by glaucoma.
Medical Management
The
aim of all glaucoma treatment is prevention of optic nerve damage through
medical therapy, laser or nonlaser surgery, or a combination of these approaches.
Lifelong therapy is almost al-ways necessary because glaucoma cannot be cured.
Although treatment cannot reverse optic nerve damage, further damage can be
controlled. The treatment goal is to maintain an IOP within a range unlikely to
cause further damage.
The
initial target for IOP among patients with elevated IOP and those with
low-tension glaucoma with progressive visual field loss is typically set at 30%
lower than the current pressure. The patient is monitored for the stability of
the optic nerve. If there is evidence of progressive damage, the target IOP is
again lowered until the optic nerve shows stability.
Treatment
focuses on achieving the greatest benefit at the least risk, cost, and
inconvenience to the patient. All treatment options have potential
complications, especially surgery, which yields the best success rates. In the
United States, medical management is the common approach, and surgical
management is the last re-sort. In Great Britain, the initial treatment of
choice is surgery (Fechtner & Singh, 2001).
PHARMACOLOGIC THERAPY
Medical
management of glaucoma relies on systemic and topical ocular medications that
lower IOP. Periodic follow-up examina-tions are essential to monitor IOP,
appearance of the optic nerve, visual fields, and side effects of medications.
In considering a therapeutic regimen, the ophthalmologist aims for the greatest
ef-fectiveness with the least side effects, inconvenience, and cost. Therapy
takes into account the patient’s health and stage of glau-coma. Comfort,
affordability, convenience, lifestyle, and person-ality are factors to consider
in the patient’s compliance with the medical regimen.
The
patient is usually started on the lowest dose of topical medication and then
advanced to increased concentrations until the desired IOP level is reached and
maintained. Because of their efficacy, minimal dosing (can be used once each
day), and low cost, beta-blockers are the preferred initial topical
medications. One eye is treated first, with the other eye used as a control in
de-termining the efficacy of the medication; once efficacy has been
established, treatment of the fellow eye is started. If the IOP is el-evated in
both eyes, both are treated. When results are not satis-factory, a new
medication is substituted. The main markers of the efficacy of the medication
in glaucoma control are lowering of the IOP to the target pressure, appearance
of the optic nerve head, and the visual field.
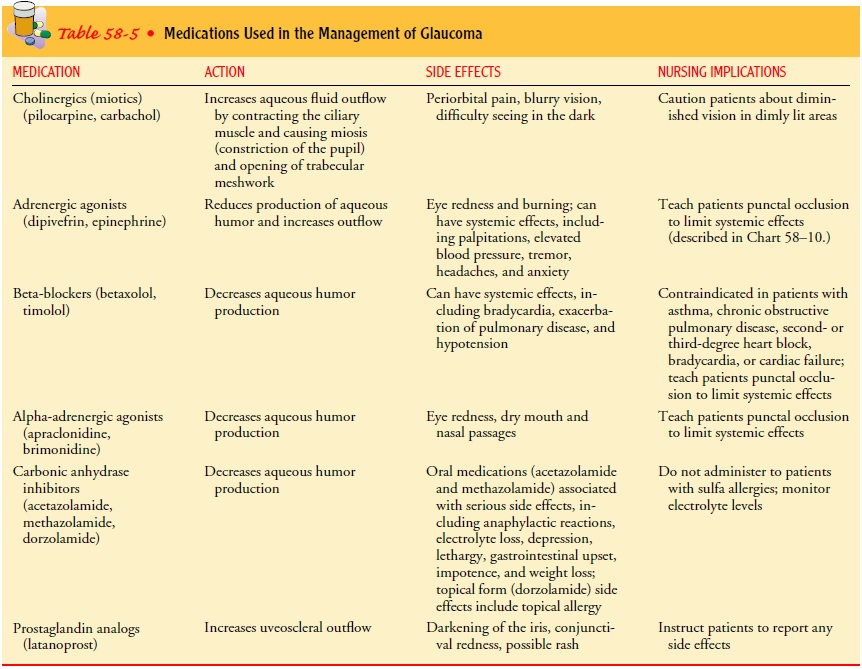
Several
types of ocular medications are used to treat glaucoma (Table 58-5), including miotics (ie, cause pupillary
constriction), adrenergic agonists (ie, sympathomimetic agents), beta-blockers,
alpha2-agonists (ie, adrenergic
agents), carbonic anhydrase in-hibitors, and prostaglandins. Cholinergics (ie,
miotics) increase the outflow of the aqueous humor by affecting ciliary muscle
con-traction and pupil constriction, allowing flow through a larger opening
between the iris and the trabecular meshwork. Adrener-gic agonists increase
aqueous outflow but primarily decrease aqueous production with an action
similar to beta-blockers and carbonic anhydrase inhibitors.
SURGICAL MANAGEMENT
In laser trabeculoplasty for glaucoma, laser burns are applied to the inner surface of the trabecular meshwork to open the intra-trabecular spaces and widen the canal of Schlemm, thereby promoting outflow of aqueous humor and decreasing IOP.
The pro-cedure is indicated when IOP is inadequately controlled by
med-ications; it is contraindicated when the trabecular meshwork cannot be
fully visualized because of narrow angles. A serious complication of this
procedure is a transient rise in IOP (usually 2 hours after surgery) that may
become persistent. IOP assess-ment in the immediate postoperative period is
essential.
In laser
iridotomy for pupillary block glaucoma, an opening is made in the iris to
eliminate the pupillary block. Laser iridotomy is contraindicated in patients
with corneal edema, which inter-feres with laser targeting and strength.
Potential complications are burns to the cornea, lens, or retina; transient
elevated IOP; closure of the iridotomy; uveitis; and blurring. Pilocarpine is
usu-ally prescribed to prevent closure of the iridotomy.
Filtering
procedures for chronic glaucoma are
used to create anopening or fistula in the trabecular meshwork to drain aqueous
humor from the anterior chamber to the subconjunctival space into a bleb,
thereby bypassing the usual drainage structures. This allows the aqueous humor
to flow and exit by different routes (ie, absorption by the conjunctival
vessels or mixing with tears). Trabeculectomy
is the standard filtering technique used to removepart of the trabecular
meshwork. Complications include hemor-rhage, an extremely low (hypotony) or
elevated IOP, uveitis, cataracts, bleb failure, bleb leak, and endophthalmitis.
Unlike other surgical procedures, the filtering procedure’s goal in glaucoma
treatment is to achieve incomplete healing of the surgical wound. The outflow
of aqueous humor in a newly created drain-age fistula is circumvented by the
granulation of fibrovascular tissue or scar tissue formation on the surgical
site. Scarring is in-hibited by using antifibrosis agents such as the
antimetabolites fluorouracil (Efudex) and mitomycin (Mutamycin). Like all
anti-neoplastic agents, they require special handling procedures before,
during, and after the procedure. Fluorouracil can be administered
intraoperatively and by subconjunctival injection during follow-up; mitomycin
is much more potent and is administered only intraoperatively.
Drainage implants or shunts are
open tubes implanted in theanterior chamber to shunt aqueous humor to an
attached plate in the conjunctival space. A fibrous capsule develops around the
episcleral plate and filters the aqueous humor, thereby regulating the outflow
and controlling IOP.
Nursing Management
TEACHING PATIENTS ABOUT GLAUCOMA CARE
The medical and surgical management of glaucoma
slows the progression of glaucoma but does not cure it. The lifelong
thera-peutic regimen mandates patient education. The nature of the disease and
the importance of strict adherence to the medication regimen must be explained
to help ensure compliance. A thor ough patient interview is essential to
determine systemic condi-tions, current systemic and ocular medications, family
history, and problems with compliance to glaucoma medications. Then the
medication program can be discussed, particularly the inter-actions of
glaucoma-control medications with other medications. For example, the diuretic
effect of acetazolamide has an additive effect on the diuretic effects of other
antihypertensive medica-tions and can result in hypokalemia. The effects of
glaucoma-control medications on vision must also be explained. Miotics and
sympathomimetics result in altered focus; therefore, patients need to be
cautious in navigating their surroundings. Informa-tion about instilling ocular
medication and preventing systemic absorption with punctal occlusion is
described in the section on ophthalmic medications.
Nurses in all settings encounter patients with
glaucoma. Even patients with long-standing disease and those with glaucoma as a
secondary diagnosis should be assessed for knowledge level and compliance with
the therapeutic regimen. Chart 58-4 contains points to review with glaucoma
patients.

CONTINUING GLAUCOMA CARE AT HOME
For patients with severe glaucoma and impaired
function, refer-ral to services that assist the patient in performing customary
ac-tivities may be needed. The loss of peripheral vision impairs mobility the
most. These patients need to be referred to low-vision and rehabilitation
services. Patients who meet the criteria for legal blindness should be offered
referrals to agencies that assist in obtaining federal assistance.
Reassurance and emotional support are important aspects of care. A lifelong disease involving a possible loss of sight has psy-chological, physical, social, and vocational ramifications.
The family must be integrated into the plan of care,
and because the disease has a familial tendency, family members should be
en-couraged to undergo examinations at least once every 2 years to detect
glaucoma early.
Related Topics