Chapter: Biotechnology Applying the Genetic Revolution: RNA-Based Technologies
Delivery of Antisense Therapies
DELIVERY
OF ANTISENSE THERAPIES
Getting antisense
oligonucleotides into cells requires special techniques, because they do not
cross cell membranes easily enough on their own to be effective. Getting the
antisense oligonucleotide into the correct cellular location poses another
obstacle to devising methods of antisense therapy. Although the uptake of
oligonucleotides occurs by an unknown mechanism, the process is active. That
is, uptake depends on temperature, the concentration of the oligonucleotide,
and the type of cell. It is thought that oligonucleotides are taken up by
endocytosis and pinocytosis. (Pinocytosis is similar to endocytosis, but the
droplets that enter the cell are much smaller.) There is also a possibility
that oligonucleotides enter via a membrane-bound receptor at very low
concentrations, whereas at higher concentrations, they enter via pinocytosis
and endocytosis.
A common method to deliver
oligonucleotides to cells is to encapsulate the oligonucleotides in liposomes (Fig. 5.8A). Liposomes are
small vesicles made of bilayers of phospholipids and cholesterol. Whether the
liposome is neutral or positively charged depends on the type of phospholipid
used to manufacture them. The oligonucleotides ride on the exterior if the
liposome is positively charged or are encapsulated inside the aqueous interior.
Positively charged liposomes are drawn to the cell surface because it is
negatively charged, and the entire
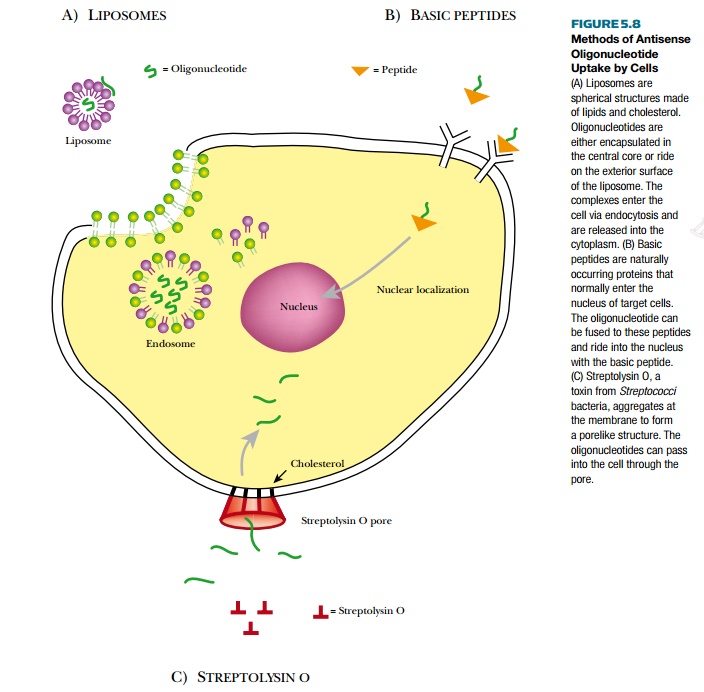
liposome, oligonucleotides
and all, is engulfed by endocytosis. Some liposomes contain “helper” molecules
that make the endosomal membrane unstable and release the liposome directly
into the cytoplasm. Other delivery “vehicles” are cationic polymers, which
include poly-L-lysine and polyethyleneimine. These operate via electrostatic
interactions as discussed earlier, but they are toxic when taken into the cell;
therefore, they are not used very often.
When the endosomal pathway is
used for uptake, as with liposomes, there is a good chance that the antisense
oligonucleotide will be degraded or not released to the cytoplasm. To alleviate
this problem, antisense oligonucleotides may be delivered to the cell attached
to basic peptides (see Fig. 5.8B).
These include the Tat protein of HIV-1, the N-terminal segment of HA2 subunit
of influenza virus agglutinin protein, and Antennapedia peptide
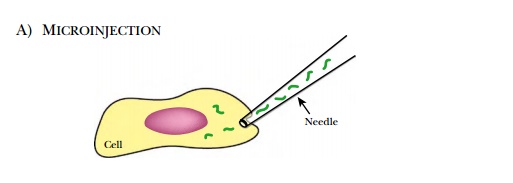
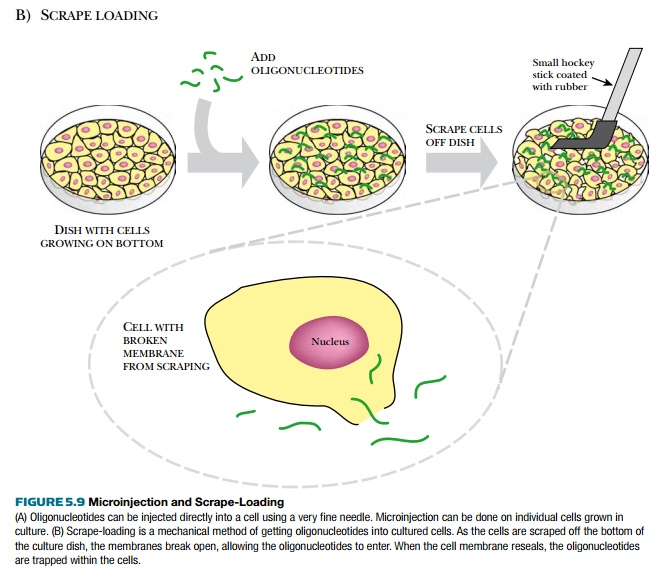
Other methods to get oligonucleotides into the cells require chemically or manually disrupting the membrane (see Fig. 5.8). Membrane pores can be generated by streptolysin O permeabilization (see Fig. 5.8C) or electroporation. Streptolysin O is a toxin from Streptococci bacteria that aggregates after binding to cholesterol in the membrane, forming a pore. The oligonucleotide passes through the pore and enters the cytoplasm directly. Antisense oligonucleotides can also be microinjected directly into each cell, but this method cannot be used for treating patients and is only useful for small-scale experiments on cultured cells (Fig. 5.9). Another mechanical method is called scrape-loading (see Fig. 5.9). Here, adherent cultured cells are gently scraped off the dish while the oligonucleotide bathes the cells. Removal of the cells probably creates small openings that allow the oligonucleotides to enter the cytoplasm.
Antisense oligonucleotides enter the target
cell by endocytosis of oligonucleotide-filled liposomes, by rid-ing on basic
peptides that normally enter the nucleus, by passing through pores created by
streptolysin O, or by mechanical shearing.
Related Topics