Chemistry - p-Block Elements-I: Choose the correct answer | 12th Chemistry : UNIT 2 : p-Block Elements-I
Chapter: 12th Chemistry : UNIT 2 : p-Block Elements-I
p-Block Elements-I: Choose the correct answer
Choose the correct answer:
1. An aqueous solution of borax is
a) neutral
b) acidic
c) basic
d) amphoteric
Solution:
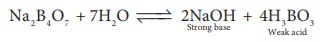
2. Boric acid is an acid because its molecule
a) contains replaceable H+ ion
b) gives up a proton
c) combines with proton to form water molecule
d) accepts OH- from water ,releasing proton.
Solution:
B(OH)3 + H2O ↔ [B (OH)4 + H+
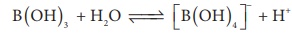
3. Which among the following is not a borane?
a) B2 H6
b) B3 H6
c) B4 H10
d) none of these
Solution:
nido borane : BnH4+n
aracno borane : BnH6+n
B3H6 is not a borane
4. Which of the following metals has the largest abundance in the earth’s crust?
a) Aluminium
b) calcium
c) Magnesium
d) sodium
5. In diborane, the number of electrons that accounts for banana bonds is
a) six
b) two
c) four
d) three
Solution:
There are two 3c – 2e-bonding in the bridges account for 4 electrons.
6. The element that does not show catenation among the following p-block elements is
a) Carbon
b) silicon
c) Lead
d) germanium
7. Carbon atoms in fullerene with formula C60 have
a) sp3 hybridised
b) sp hybridised
c) sp2 hybridised
d) partially sp2 and partially sp3 hybridised
8. Oxidation state of carbon in its hydrides
a) +4
b) -4
c) +3
d) +2
Solution:
Example : CH4+ in which the oxidation state of carbon is +4
9. The basic structural unit of silicates is
a) ( SiO3 )2−
b) ( SiO4 )2−
c) ( SiO)−
d) ( SiO4 )4−
10. The repeating unit in silicone is
a) SiO2
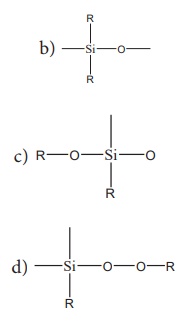
Ans: b
11. Which of these is not a monomer for a high molecular mass silicone polymer?
a) Me3SiCl
b) PhSiCl3
c) MeSiCl3
d) Me2SiCl2
12. Which of the following is not sp2 hybridised?
a) Graphite
b) graphene
c) Fullerene
d) dry ice
Solution:
dry ice – solid CO2 in which carbon is in sp hybridized state
13. The geometry at which carbon atom in diamond are bonded to each other is
a) Tetrahedral
b) hexagonal
c) Octahedral
d) none of these
14. Which of the following statements is not correct?
a) Beryl is a cyclic silicate
b) Mg2SiO4 is an orthosilicate
c) SiO4 4−is the basic structural unit of silicates
d) Feldspar is not aluminosilicate
15. AlF3 is soluble in HF only in the presence of KF. It is due to the formation of
a) K3 [ AlF3H3 ]
b) K3 [ AlF6 ]
c) AlH3
d) K[ AlF3H]
Solution:
AlF3 + 3KF → K3[AlF6]
16. Match items in column - I with the items of column – II and assign the correct code.
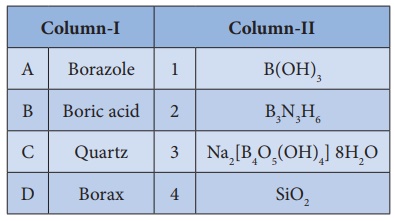
a) A-2 , B-1 , C-4 , D-3
b) A-1 , B-2 , C-4 , D-3
c) A-1 , B-2 , C-4 , D-3
d) none of these
17. Duralumin is an alloy of
a) Cu,Mn
b) Cu,Al,Mg
c) Al,Mn
d) Al,Cu,Mn,Mg
Solution:
Al-95% , Cu-4% , Mn-0.5% , Mn-0.5%
18. Thermodynamically the most stable form of carbon is
a) Diamond
b) graphite
c) Fullerene
d) none of these
19. The compound that is used in nuclear reactors as protective shields and control rods is
a) Metal borides
b) metal oxides
c) Metal carbonates
d) metal carbide
20. The stability of +1 oxidation state increases in the sequence
a) Al < Ga < In < Tl
b) Tl < In < Ga < Al
c) In < Tl < Ga < Al
d) Ga< In < Al < Tl
Solution
stability of +1 oxidation state decreases down the group due to inert pair effect
PTA Question Oneword:
1. The basic structural unit of
silicates is
a)
[SiO3]2−
b)
[SiO4]2−
c)
[SiO]−
d) [SiO4]4−
Answer: d)
2. Assertion : Aqueous solution
of potash Alum is acidic.
Reason: Aluminium sulphate
undergo hydrolysis.
a) Both assertion and reason are
true and reason is the correct explanation of assertion
b)
Both assertion and reason are true but reason is not the correct explanation of
assertion
c)
Assertion is true but reason is false
d)
Both assertion and reason are false
Answer: a)
3. Ortho boric acid on
dehydration at 373K produces mainly
a) metaboric acid
b)
boric anhydride
c)
Boron metal and oxygen
d)
tetra boric acid
Answer: a)
4. Which of the following
statement about H3BO3 is not correct?
a) It is a strong tribasic acid
b)
It is prepared by acidifying an aqueous solution of borax.
c)
It is a layer structure in which planer BO3 units are joined by
hydrogen bonds.
d)
It does not act as proton donor but acts as a Lewis acid by accepting hydroxyl
ion.
Answer: a)
5. On hydrolysis BF3 gives
Boric acid and converted to fluroboric acid. The fluoroboric acid contains the
species.
a)
H+, F− & BF3
b)
H+ & [BF4] −
c)
H, BF3]+ & F−
d)
H+, B3+ & F−
Answer: b)
Related Topics