Preparation, Properties, Structure, Uses - Boron trifluoride | 12th Chemistry : UNIT 2 : p-Block Elements-I
Chapter: 12th Chemistry : UNIT 2 : p-Block Elements-I
Boron trifluoride
Boron
trifluoride:
Preparation:
Boron trifuloride is
obtained by the treatment of calcium fluoride with boron trioxide in presence
of conc. sulphuric acid.

It can also be obtained
by treating boron trioxide with carbon and fluorine.
B2O3
+ 3C + 3F2 → 2BF3
+ 3CO
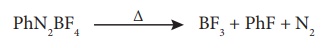
In the laboratory pure
BF3 is prepared by the thermal decomposition of benzene diazonium
tetrafluoro borate.
Properties:
Boron trifluoride has a
planar geometry. It is an electron deficient compound and accepts electron
pairs to form coordinate covalent bonds. They form complex of the type [BX4]-.
BF3 + NH3
→ F3 ← B NH3
BF3 + H2O
→ F3B ← OH2
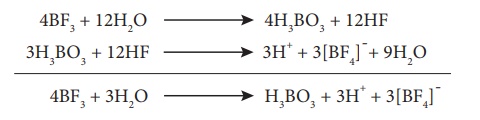
On hydrolysis, boric
acid is obtained. This then gets converted into fluoro boric acid.
Uses of Boron trifluoride:
·
Boron trifluoride is used for preparing HBF4, a
catalyst in organic chemistry
·
It is also used as a fluorinating reagent.
Related Topics