Preparation, Properties, Uses - Silicon tetrachloride | 12th Chemistry : UNIT 2 : p-Block Elements-I
Chapter: 12th Chemistry : UNIT 2 : p-Block Elements-I
Silicon tetrachloride
Silicon
tetrachloride:
Preparation:
Silicon tetrachloride
can be prepared by passing dry chlorine over an intimate mixture of silica and
carbon by heating to 1675 K in a porcelain tube
SiO2 + 2C +
2Cl2 → SiCl4 + 2CO
On commercial scale,
reaction of silicon with hydrogen chloride gas occurs above 600 K
Si + 4HCl → SiCl4 + 2H2
Properties:
Silicon tetrachloride is
a colourless fuming liquid and it freezes at -70 ⁰C
In moist air, silicon
tetrachloride is hydrolysed with water to give silica and hydrochloric acid.
SiCl4 + 4H2O
→ 4HCl + Si(OH)4
When silicon
tetrachloride is hydrolysed with moist ether, linear perchloro siloxanes are
formed [Cl-(Si Cl2O)nSiCl3 where n=1-6.
Alcoholysis
The chloride ion in
silicon tetrachloride can be substituted by nucleophile such as OH, OR, etc..
using suitable reagents. For example, it forms silicic esters with alcohols.
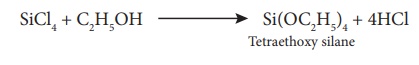
SiCl4 + C2H5OH
→ 4HCl + Si(OC2H5)4 Tetraethoxy silane
Ammonialysis.
Similarly silicon
tetrachloride undergoes ammonialysis to form chlorosilazanes.

Uses:
·
Silicon tetrachloride is used in the production of semiconducting
silicon.
·
It is used as a starting material in the synthesis of silica gel,
silicic esters, a binder for ceramic materials.
Related Topics