Preparation, Properties, Types, Uses - Silcones | 12th Chemistry : UNIT 2 : p-Block Elements-I
Chapter: 12th Chemistry : UNIT 2 : p-Block Elements-I
Silcones
Silcones:
Silicones or poly
siloxanes are organo silicon polymers with general empirical formula (R2SiO).
Since their empirical formula is similar to that of ketone (R2CO),
they were named “silicones”. These silicones may be linear or cross linked.
Because of their very high thermal stability they are called high –temperature
polymers.
Preparation:
Generally silicones are
prepared by the hydrolysis of dialkyldichlorosilanes (R2SiCl2)
or diaryldichlorosilanes Ar2SiCl2, which are prepared by
passing vapours of RCl or ArCl over silicon at 570 K with copper as a catalyst.
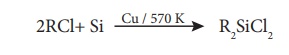
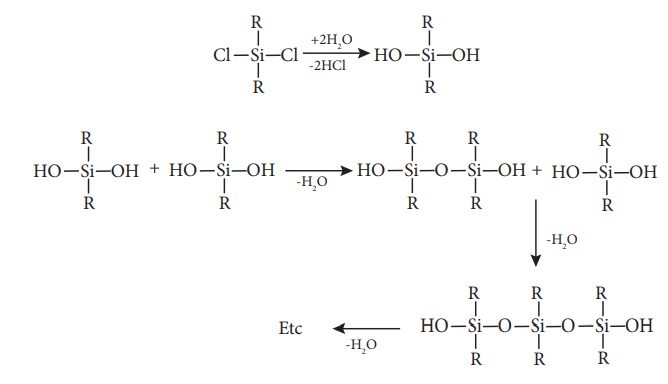
The hydrolysis of
dialkylchloro silanes R2SiCl2 yields to a straight chain
polymer which grown from both the sides
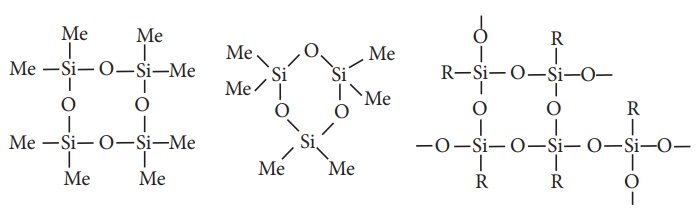
The hydrolysis of
monoalkylchloro silanes RSiCl3 yields to a very complex cross linked
polymer.. Linear silicones can be converted into cyclic or ring silicones when
water molecules is removed from the terminal –OH groups.
Types of silicones:
(i) Liner silicones:
They are obtained by the
hydrolysis and subsequent condensation of dialkyl or diaryl silicon chlorides.
1.
Silicone rubbers: These silicones are bridged together by
methylene or similar groups
2.
Silicone resins: They are obtained by blending silicones with
organic resins such as acrylic esters.
(ii) Cyclic silicones
These are obtained by
the hydrolysis of R2SiCl2.
(iii) Cross linked silicones
They are obtained by
hydrolysis of RSiCl3
Properties
The extent of cross
linking and nature of alkyl group determine the nature of polymer. They range
from oily liquids to rubber like solids. All silicones are water repellent.
This property arises due to the presence of organic side groups that surrounds
the silicon which makes the molecule looks like an alkane. They are also
thermal and electrical insulators. Chemically they are inert. Lower silicones
are oily liquids whereas higher silicones with long chain structure are waxy
solids. The viscosity of silicon oil remains constant and doesn’t change with
temperature and they don't thicken during winter
Uses:
·
Silicones are used for low temperature lubrication and in vacuum
pumps, high temperature oil baths etc...
·
They are used for making water proofing clothes
·
They are used as insulting material in electrical motor and other
appliances
·
They are mixed with paints and enamels to make them resistant
towards high temperature, sunlight, dampness and chemicals.
Related Topics