Occurrence, Physical properties, Chemical properties, Uses of boron - Group 13 (Boron group) elements | 12th Chemistry : UNIT 2 : p-Block Elements-I
Chapter: 12th Chemistry : UNIT 2 : p-Block Elements-I
Group 13 (Boron group) elements
Group
13 (Boron group) elements:
Occurrence:
The boron occurs mostly
as borates and its important ores are borax - Na2[B4O5(OH)4].8H2O
and kernite - Na2[B4O5(OH)4].2H2O..
Aluminium is the most abundant metal and occurs as oxides and also found in
aluminosilicate rocks. Commercially it is extracted from its chief ore, bauxite
(Al2O3.2H2O). The other elements of this group
occur only in trace amounts. The other elements Ga, In and Tl occur as their
sulphides.
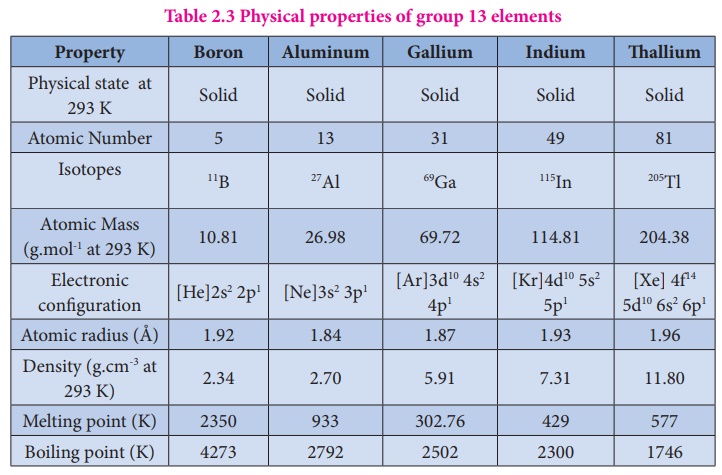
Physical properties:
Some of the physical
properties of the group 13 elements are listed below
Table 2.3 Physical properties of group 13 elements
Chemical properties of boron:
Boron is the only
nonmetal in this group and is less reactive. However, it shows reactivity at
higher temperatures. Many of its compounds are electron deficient and has
unusual type of covalent bonding which is due to its small size, high
ionisation energy and similarity in electronegativity with carbon and hydrogen.
Formation of metal borides:
Many metals except
alkali metals form borides with a general formula MxBy (x
ranging upto 11 and y ranging upto 66 or higher)
Direct combination of metals with boron:
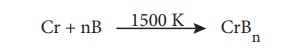
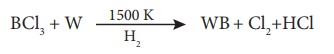
Reduction of borontrihalides:
Reduction of
borontrichloride with a metal assisted by dihydrogen gives metal borides.
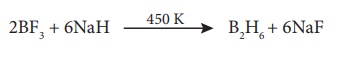
Formation of hydrides:
Boron does not react
directly with hydrogen. However, it forms a variety of hydrides called boranes.
The simplest borane is diborane - B2H6. Other larger
boranes can be prepared from diborane. Treatment of gaseous boron trifluoride
with sodium hydride around 450 K gives diborane. To prevent subsequent
pyrolysis, the product diborane is trapped immediately.
Formation of boron trihalides:
Boron combines with
halogen to form boron trihalides at high temperatures.
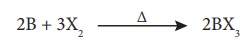
Formation of boron nitride:
Boron burns with
dinitrogen at high temperatures to form boron nitride.
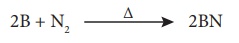
Formation of oxides:
When boron is heated
with oxygen around 900 K, it forms its oxide.
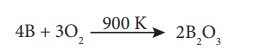
Reaction with acids and alkali:
Halo acids have no
reaction with boron. However, boron reacts with oxidising acids such as
sulphuric acid and nitric acids and forms boric acid.
2B + 3H2SO4
→ 2H3BO3 + 3SO2
B + 3HNO3 → H3BO3 + 3NO2
Boron reacts with fused
sodium hydroxide and forms sodium borate.
2B + 6NaOH → 2Na3BO3 + 3H2
Uses of boron:
·
Boron has the capacity to absorb neutrons. Hence, its isotope 10B5
is used as moderator in nuclear reactors.
·
Amorphous boron is used as a rocket fuel igniter.
·
Boron is essential for the cell walls of plants.
·
Compounds of boron have many applications. For example eye drops,
antiseptics, washing powders etc.. contains boric acid and borax. In the
manufacture of Pyrex glass , boric oxide is used.
Related Topics