Preparation, Properties, Uses - Aluminium chloride | 12th Chemistry : UNIT 2 : p-Block Elements-I
Chapter: 12th Chemistry : UNIT 2 : p-Block Elements-I
Aluminium chloride
Aluminium
chloride:
Preparation:
When aluminium metal or
aluminium hydroxide is treated with hydrochloric acid, aluminium trichloride is
formed. The reaction mixture is evaporated to obtain hydrated aluminium
chloride.
2Al + 6HCl → 2AlCl3
+ 3H2
Al(OH)3 +
3HCl → AlCl3 + 3H2O
McAfee Process:
Aluminium chloride is
obtained by heating a mixture of alumina and coke in a current of chlorine.
Al2O3
+3C + 3Cl2 → 2AlCl3
+ 3CO2
On industrial scale it
is prepared by chlorinating aluminium around 1000 K
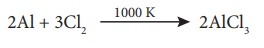
Properties:
Anhydrous aluminium
chloride is a colourless, hygroscopic substance.
An aqueous solution of
aluminium chloride is acidic in nature. It also produces hydrogen chloride
fumes in moist air.
AlCl3 + 3H2O
→ Al(OH)3 + 3HCl
With ammonium hydroxide
it forms aluminium hydroxide.
AlCl3 + 3NH4OH
→ Al(OH)3 + 3NH4Cl
With excess of sodium
hydroxide it produces metal aluminate
AlCl3 + 4NaOH
→ NaAlO2 + 2H2O + 3NaCl
It behaves like a Lewis
acid and forms addition compounds with ammonia, phosphine and carbonylchloride
etc... Eg. AlCl3.6NH3.
Uses of aluminium chloride:
·
Anhydrous aluminium chloride is used as a catalyst in Friedels
Crafts reactions
·
It is used for the manufacture of petrol by cracking the mineral
oils.
·
It is used as a catalyst in the manufacture on dyes, drugs and
perfumes.
Related Topics