Book Back and Important Questions Answers | Chemistry - p-Block Elements-I: Answer the following questions | 12th Chemistry : UNIT 2 : p-Block Elements-I
Chapter: 12th Chemistry : UNIT 2 : p-Block Elements-I
p-Block Elements-I: Answer the following questions
Chemistry : p-Block Elements-I
Answer the following questions:
1. Write a short note on anamolous properties of the first element of p-block.
Anamolous
properties are due to
1.
Small size of the first element
2.
High ionisation enthalpy and high electronegativity
3.
Absence of d orbitals in their
valence shell
2. Describe briefly allotropism in p- block elements with specific reference to carbon.
Carbon
exhibits the following allotropic form
(i)
Graphite
(ii)
Diamond
(iii)
Fullerene
(iv)
Carbon nanotubes
(v)
Graphene
(i) Graphite
• It is More stable form, soft and
conducts electricity.
• It is composed of flat two
dimensional sheets of carbon atoms. Each sheet is a hexagonal and posses sp2
hybridised carbon atoms. The C - C bond length is 1.41 Ǻ
• Each carbon atom forms three σ
bonds and the fourth electron present in the unhybridised p orbital is free and
responsible for conductivity.
• The successive carbon sheets are
held together by weak van der Waals forces. The distance between two successive
layers is 3.40 Ǻ.
• It is used as a lubricant.
(ii) Diamond
• Diamond is very hard.
• sp3 hybridised and
bonded to four neighbouring carbon atoms by σ bonds.
• It has a tetrahedral arrangement
in the entire lattice. C - C bond length is 1.54 Ǻ.
• Since all four valence electrons
of carbon are involved in bonding there is no free electrons for conductivity.
• It used for sharpening hard
tools, cutting glasses, making bores and rock drilling.
(iv) Fullerenes
• These allotropes are discrete
molecules such as C32, C50, C60, C70,
C76 etc.
• These molecules have cage like
structures.
• C60 molecule has 20
six membered rings and 12 five membered rings.
• Each carbon atom is sp2
hybridised and has a delocalised π bond giving aromatic character to these
molecules. C - C bond distance is 1.44 Ǻ.
(iv) Carbon nanotubes
• It have graphite like tubes with
fullerence ends.
• Nanotubes are stronger than steel
and conduct electricity.
• It is applied in nanoscale
electronics, catalysis, polymers and medicine.
(v) Graphene
It
has a single planar sheet of sp2 hybridised carbon atoms that are
densely packed in a honeycomb crystal lattice.
3. Boron does not react directly with hydrogen. Suggest one method to prepare diborane from BF3.
Boron
does not react directly with hydrogen. Treatment of gaseous boron trifluoride
with sodium hydride around 450 K gives diborane.
2BF3
+ 6NaH __ 450 K→ B2H6 + 6NaF
4. Give the uses of Borax.
1.
Identification of coloured metal ions.
2.
Manufacture of optical and borosilicate glass, enamels and glazes for pottery
3.
Used as a flux in metallurgy
4.
Food preservatives
5. What is catenation ? describe briefly the catenation property of carbon.
Catenation
is an ability of an element to form chain of atoms.
Carbon
possesses the following properties and forms a wide range of compounds with
itself.
(i)
The valency of element is greater than or equal to two
(ii)
Element should have an ability to bond with itself
(iii)
The self bond must be as strong as its bond with other elements
(iv)
Kinetic inertness of catenated compound towards other molecules.
6. Write a note on Fisher tropsch synthesis.
Carbon
monoxide reacts with hydrogen at a pressure of less than 50 atm using metal
catalysts at 500 - 700 K yields saturated and unsaturated hydrocarbons is known
as Fisher tropsch synthesis.
nCO
+ (2n+l)H2 → CnH(2n+2) + nH2O
nCO
+ 2nH2 → CnH2n + nH2O
7. Give the structure of CO and CO2.
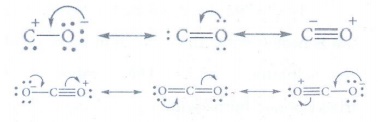
8. Give the uses of silicones.
1.
Silicones are used for low temperature lubrication and in vacuum pumps, high
temperature oil baths.
2.
They are used for making water proofing clothes
3.
They are used as insulting material in electrical motor and other appliances
4.
They are mixed with paints and enamels to make them resistant towards high
temperature, sunlight, dampness and chemicals.
9. AlCl3 behaves like a lewis acid. Substantiate this statement.
AlCl3
behaves like a Lewis acid and forms addition compounds with ammonia, phosphine and
carbonylchloride.
Eg.
AlCl3.6NH3.
10. Describe the structure of diborane.
• Two BH2 units are
linked by two bridged hydrogens with eight B - H bonds.
• Diborane has only 12 valence electrons
and are not sufficient to form normal covalent bonds.
• 8 electrons used for 2c-2e bonds
and the remaining four elections used for 3c-2e bond.
• The boron is sp3
hybridised. Three of the four sp3 hybridised orbitals contains
single electron and the fourth orbital is empty.
• Two of the half filled hybridised
orbitals of each boron overlap with the two hydrogens to form four terminal
2c-2e bonds, leaving one empty and one half filled hybridised orbitals on each
boron.
• The bridging hydrogen atoms are
in a plane.
• The three centre - two electron
bonds, involves overlapping the half filled hybridised orbital of one boron,
the empty hybridised orbital of the other boron and the half filled is orbital
of hydrogen.
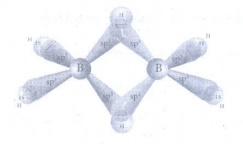
11. Write a short note on hydroboration.
Diborane
adds on to alkenes and alkynes in ether solvent at room temperature is called
hydroboration. It is highly used in synthetic organic chemistry, especially for
anti Markovnikov addition.
B2H6
+ 3RCH = CHR → 2B(CH2CH2R)3 + 6H2
12. Give one example for each of the following
(i) icosogens (ii) tetragen (iii) prictogen (iv) chalcogen
(i)
Icosogens (ii) Tetragen (iii) Pnictogen (iv) Chalcogen
(i)
Icosogens - Boron
(ii)
Tetragen - Carbon
(iii)
Pnictogen - Nitrogen
(iv)
Chalcogen - Oxygen
13. Write a note on metallic nature of p-block elements.
• The tendency of an element to
form a cation by loosing electrons is known as metallic character. On
descending a group the ionisation energy decreases and hence the metallic
character increases.
• The elements present in lower
left part are metals while the elements in the upper right part are non metals.
• Due to higher ionisation energy
all the elements of group 17 and 18 are non metals.
• Higher nuclear charge is
responsible for nonmetallic character of boron.
14. Complete the following reactions
a. B ( OH)3 +NH3 →
b. Na2B4O7 + H2SO4 + H2O →
c. B2H6 + 2NaOH + 2H2O →
d. B2H6 + CH3OH →
e. BF3 + 9 H2O →
f . HCOOH + H2SO4 →
g . SiCl4 + NH3 →
h. SiCl4 + C2H5OH →
i. B + NaOH →
j. H2B4O7 Red hot→
Answer:
a)
B(OH)3 + NH3 ___Δ→ BN+3H2O
b)
Na2B4O7 + H2SO4 + 5H2O
→ 4 H3BO3 + Na2SO4
c)
B2H6 + 2NaOH + 2H2O → 2NaBO2 + 6H2
d)
B2H6 + 6CH3OH → 2B(OCH3)3 +
6H2
e)
BF3 + H2O → F3B ← OH2
f)
HCOOH + H2SO4 → CO + H2O. H2SO4
g)
2SiCl4 + NH3 ___33Ok_Ether__→
Cl3SiNHSiCl3 + 2HCl
h)
SiCl4 + 4C2H5OH → Si(OC2H5)4
+ 4HCl
i)
2B + 6NaOH → 2Na3BO3 + 3H2
j)
H2B4O7 ___Red box → 2B2O3
+ H2O
15. How will you identify borate radical?
Trialkylborate
is formed when borate salt is heated with ethyl alcohol in presence of
conc.sulphuric acid. The vapour of trialkylborate burns with a green edged
flame and this reaction is used to identify the presence of borate.
H3BO3
+ 3C2H5OH ___Conc. H2SO4__→ B(OC2H5)3
+ 3H2O
16. Write a note on zeolites.
• Zeolites are three-dimensional
crystalline solids containing aluminium, silicon, and oxygen in their regular three
dimensional framework. They are hydrated sodium alumino silicates with general
formula Na2O.(Al2O3).x(SiO2).yH2O
(x=2
to 10; y=2 to 6)
• Zeolites have porous structure in
which the monovalent sodium ions and water molecules are loosely held. The Si
and Al atoms are tetrahedrally coordinated with each other through shared
oxygen atoms.
• Zeolites have a three dimensional
crystalline structure consisting of a network of interconnected tunnels and
cages. Water molecules moves freely in and out of these pores but the zeolite
framework remains rigid.
• The pore /channel sizes are
nearly uniform, allowing the crystal to act as a molecular sieve.
17. How will you convert boric acid to boron nitride?
Fusion
of urea with boric acid in ammonia atmosphere at 800 −1200 K gives boron
nitride
B(OH)3
[Boric acid] + NH3 ____Δ__→
BN [Boron nitride] + 3H2O
18. A hydride of 2nd period alkali metal (A) on reaction with compound of Boron (B) to give a reducing agent (C). identify A , B and C.
Alkali
metal present in second period is Li and its hydride is LiH(A). LiH(A) reacts
with boron compound B2H6(B) gives a reducing agent LiBH4
(C).
2LiH + B2H6 ___Ether_→ 2LiBH4
(A) (B) (C)
A
: LiH - Lithium hydride
B
: B2H6 - Diborane
C
: LiBH4 - Lithium borohydride
19. A double salt which contains fourth period alkali metal (A) on heating at 500K gives (B). aqueous solution of (B) gives white precipitate with BaCl2 and gives a red colour compound with alizarin. Identify A and B.
Alkali
metal present in fourth period is potassium. Its double salt is potash alum.
K2SO4.
Al2(SO4)3. 24H2O
A
is K2SO4. Al2(SO4)3. 24H2O
A
on heating at 500 K gives burnt alum.
K2SO4 Al2(SO4)3.
24 H2O ___500 K_→ K2SO4.Al2(SO4)3
+ 24 H2O
Potash Alum (A) Burnt Alum (B)
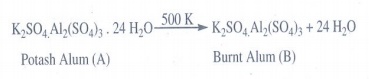
In
aqueous solution B ionizes and gives sulphate ion. While adding BaCl2,
sulphate ion reacts with BaCl2 gives BaSO4 precipitate
which is white colour. B also gives red colour with alizarin due to the
presence of Al3+.
A
: K2SO4. Al2(SO4)3. 24 H2O
- Potash alum
B
: K2SO4. Al2(SO4)3 - Burnt
alum
20. CO is a reducing agent . justify with an example.
Carbon
monoxide acts as a strong reducing agent. It reduces iron oxide into iron.
3CO
+ Fe2O3 ___Heat__→ 2Fe + 3CO2
Related Topics