Group 14 (Carbon group) elements | p-Block Elements-I | Chemistry - Silicates | 12th Chemistry : UNIT 2 : p-Block Elements-I
Chapter: 12th Chemistry : UNIT 2 : p-Block Elements-I
Silicates
Silicates
The mineral which
contains silicon and oxygen in tetrahedral [SiO4]4- units
linked together
in different patterns are called silicates. Nearly 95 % of the earth crust is
composed of silicate minerals and silica. The glass and ceramic industries are
based on the chemistry silicates.
Types of Silicates:
Silicates are classified
into various types based on the way in which the tetrahedral units, [SiO ]4-4
are linked together.
Ortho silicates (Neso
silicates): The simplest silicates which contain discrete [SiO4]4-
tetrahedral units are called ortho silicates or neso silicates.
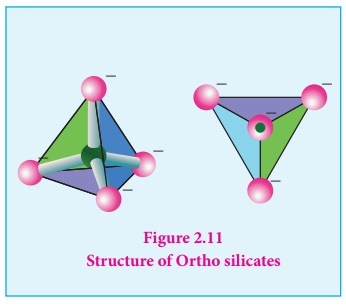
Examples : Phenacite - Be2SiO4
(Be2+ ions are tetrahedrally surrounded by O2-
ions), Olivine - (Fe/Mg)2SiO4 ( Fe2+
and Mg2+ cations are octahedrally surrounded by O2-
ions),
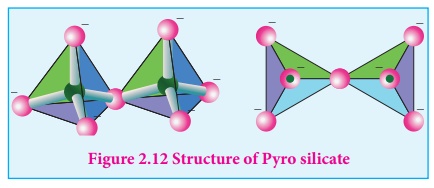
Pyro silicate (or) Soro silicates):
Silicates which contain [Si2O7]6-
ions are called pyro silicates (or) Soro silicates. They are formed by joining two
[SiO4]4- tetrahedral units by sharing one oxygen atom at one
corner.(one oxygen is removed while joining). Example : Thortveitite - Sc2Si2O7
Cyclic silicates (or Ring silicates)
Silicates which contain
(SiO3)n2n- ions which are formed by linking
three or more tetrahedral SiO44- units cyclically are
called cyclic silicates. Each silicate unit shares two of its oxygen atoms with
other units.
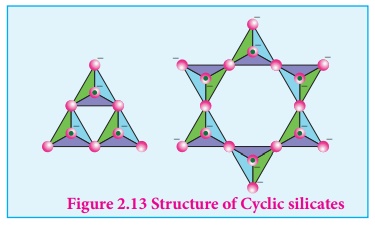
Example: Beryl [Be3Al2 (SiO3)6] (an aluminosilicate with each aluminium is surrounded by 6 oxygen atoms octahedrally)
Inosilicates :
Silicates which contain
'n' number of silicate units liked by sharing two or more oxygen atoms are
called inosilicates. They are further classified as chain silicates and double
chain silicates.
Chain silicates (or pyroxenes): These silicates contain [(SiO3) n]2n-
ions formed b linking ‘n’ number of tetrahedral [SiO4]4- units
4 linearly. Each silicate unit shares two of its oxygen atoms with other units.
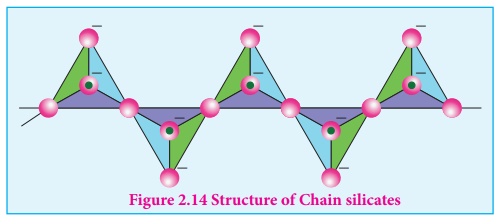
Example: Spodumene - LiAl(SiO3)2.
Double chain silicates
(or amphiboles): These silicates contains [Si4O11]n6n-
ions. In these silicates there are two different types of tetrahedra : (i)
Those sharing 3 vertices (ii) those sharing only 2 vertices.
Examples:
1) Asbestos : These are Fibrous and non- combustible silicates.
Therefore they are used
for thermal insulation material, brake linings, construction material and filters.
Asbestos being carcinogenic silicates, their applications are restricted.
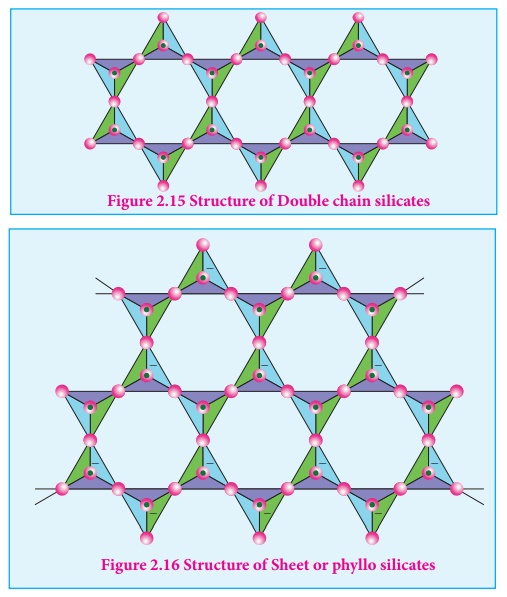
Sheet or phyllo silicates
Sheet or phyllo
silicates Silicates which contain (Si2O5)n2n-
are called sheet or phyllo silicates. In these, Each [ SiO4 ] 4
- tetrahedron unit shares three oxygen atoms with others and thus by forming two-
dimensional sheets. These sheets silicates form layered structures in which
silicate sheets are stacked over each other. The attractive forces between
these layers are very week, hence they can be cleaved easily just like
graphite.
Example: Talc, Mica etc...
Three dimensional silicates (or tecto silicates):
Silicates in which all the oxygen atoms of [SiO4]4-tetrahedra are shared with other tetrahedra to form three-dimensional network are called three dimensional or tecto silicates. They have general formula (SiO2)n .
Examples: Quartz
These tecto silicates can be converted into Three dimentional aluminosilicates by replacing [SiO4]4- units by [AlO4]5- units. E.g. Feldspar, Zeolites etc.,
Related Topics