Preparation, Properties, Uses of Borax - Borax | 12th Chemistry : UNIT 2 : p-Block Elements-I
Chapter: 12th Chemistry : UNIT 2 : p-Block Elements-I
Borax
Borax
[Na2B4O7.10H2O]:
Preparation:
Borax is a sodium salt
of tetraboric acid. It is obtained from colemanite ore by boiling its solution
with sodium carbonate.

Borax is normally
formulated as Na2B4O7.10H2O. But it
contains, tetranuclear units [B4O5. (OH)4]2-.
This form is known as prismatic form. Borax also exists two other forms namely,
jeweller or octahderal borax (Na2B4O7.5H2O)
and borax glass (Na2B4O7).
Properties
Borax is basic in nature
and its solution in hot-water is alkaline as it dissociates into boric acid and
sodium hydroxide.
Na2B4O7
+ 7H2O → 4H3BO3 + 2NaOH
On heating it forms a
transparent borax beads.
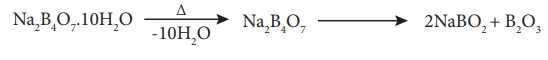
Borax reacts with acids
to form sparingly soluble boric acid.
Na2B4O7
+ 2HCl + 7H2O → 4H3BO3 + 2NaCl
Na2B4O7
+ H2SO4 + 5H2O → 4H3BO3 + 2Na2SO4
When treated with ammonium
chloride it forms boron nitride.
Na2B4O7
+ 2NH4Cl → 2NaCl + 2BN + B2O3
+ 4H2O
Uses of Borax:
·
Borax is used for the identification of coloured metal ions
·
In the manufacture optical and borosilicate glass, enamels and
glazes for pottery
·
It is also used as a flux in metallurgy and also acts as a
preservative
Related Topics