Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
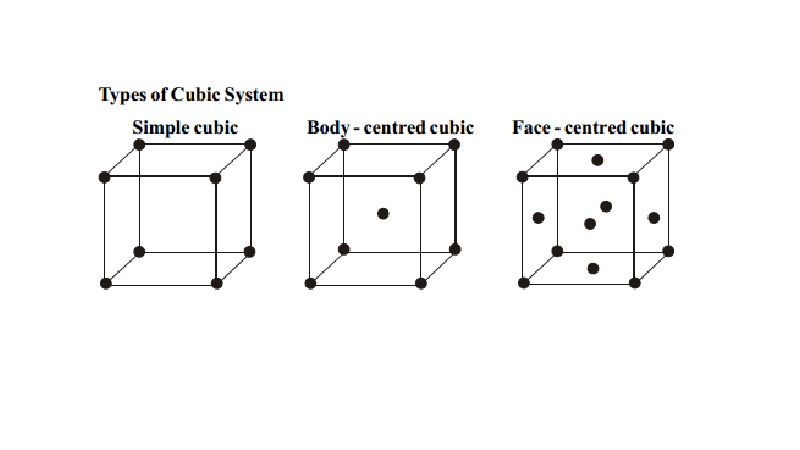
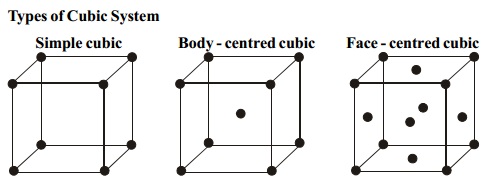
Types of Cubic System
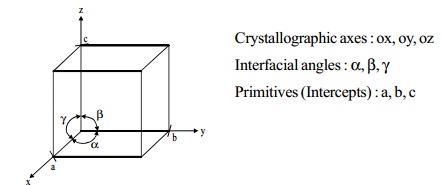
UNIT CELL
Unit cell is the smallest fundamental repeating portion
of a crystal lattice from which the
crystal is built by repetition in three dimension.
Characteristic parameters of a unit cell
Crystallographic axes : ox, oy, oz
Interfacial angles : a, b, g
Primitives (Intercepts) : a, b, c
Types of Cubic System
i.
Simple cubic
ii.
Body - centred
cubic
iii.
Face - centred
cubic
Assignment of Atoms
per unit cell in a cubic lattice
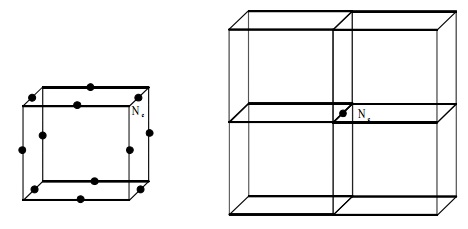
Simple Cubic
In a simple cubic where atoms are present at the corners
only, each atom at the corner is
shared equally by eight other unit cells. Hence the contribution of each atom
to the unit cell is 1/8.
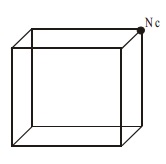
The total number of atoms per
unit cell = Nc /8 = 8/8 = 1
Nc is the number of atoms at
the corners.
Fcc
A face atom is shared equally
between two unit cells and therefore a face atom
contributes only (Nf/2) to the unit cell.
The number of atoms per unit
cell in fcc = Nc/8 + Nf/2 = (8/8) + (6/2) = 4
Nf = Number of atoms at the faces.
Nf = Number of atoms
at the faces
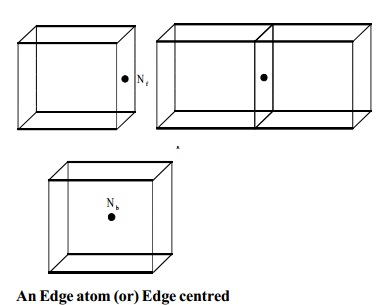
BCC
In a bcc lattice, the body
centred atom belongs exclusively to the unit cell.
The total number of atoms per unit cell in bcc
= (Nc/8) + (Ne/4)
= (8/8) + (12/4) = 4
Nb = Number of atoms inside
the body
An Edge atom (or) Edge centred
An edge atom and edge centred is common to four unit cells and there are twelve edges of the unit cell. The contribution from each edge atom is therefore 1/4. The number of atoms per unit cell in edge centre.
= (Nc/8) + (Ne/4)
= (8/8) + (12/4) = 4
Ne = Number of atoms at the
edge centre
Related Topics