Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Calculation of Empirical Formula from Quantitative Analysis and Percentage composition
Calculation of
Empirical Formula from Quantitative Analysis and Percentage composition
Empirical Formula
"An empirical formula (or) simplest formula for a compound is the
formula of a substance written with the smallest integer subscripts".
For most ionic substances, the empirical formula
is the formula of the compound. This is often not the case for molecular
substances. For example, the formula of sodium peroxide, an ionic compound of
Na+ and O22-, is Na2O2.
Its empirical formula is NaO. Thus empirical formula tells you the ratio of
numbers of atoms in the compound.
Steps for writing the
Empirical formula
The percentage of the elements in the compound is determined by suitable
methods and from the data collected, the empirical formula is determined by the
following steps.
1.
Divide the percentage of each element by its
atomic mass. This will give the relative number of moles of various elements
present in the compound.
2.
Divide the quotients obtained in the above step
by the smallest of them so as to get a simple ratio of moles of various
elements.
3.
Multiply the figures, so obtained by a suitable
integer of necessary in order to obtain whole number ratio.
4.
Finally write down the symbols of the various
elements side by side and put the above numbers as the subscripts to the lower
right hand of each symbol. This will represent the empirical formula of the
compound.
Solved Problem
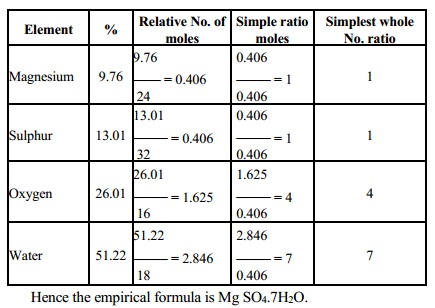
1.A compound has the
following composition Mg = 9.76%,S = 13.01%, 0 = 26.01, H2O = 51.22, what is
its empirical formula?
[Mg = 24, S = 32, O = 16, H = 1]
Solution
Hence the empirical formula is Mg SO4.7H2O.
Molecular Formula from
Empirical Formula
The molecular formula of a compound is a multiple of its empirical
formula.
Example
1.The molecular formula of acetylene, C2H2 is equivalent to (CH)2, and
the molecular formula of benzene, C6H6 is equivalent to
(CH)6. Therefore, the molecular weight is some multiple of the
empirical formula weight, which is obtained by summing the atomic Weights from
the empirical formula. For any molecular compound.
Molecular Weight = n x empirical formula weight.
Where `n' is the whole number of empirical formula units in the
molecule. The molecular formula can be obtained by multiplying the subscripts
of the empirical formula by `n' which can be calculated by the following
equation
n = Molecular Weight / Empirical formula Weight
Solved Problem
1. A
compound on analysis gave the following percentage composition C = 54.54%, H,
9.09% 0 = 36.36. The vapour density of the compound was found to be 44. Find
out the molecular formula of the compound.
Solution
Calculation of empirical formula
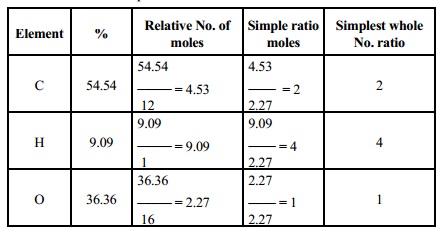
Empirical formula is C2 H4 O.
Calculation of
Molecular formula
Empirical formula mass = 12 x 2 + 1 x 4 + 16 x 1 = 44
Molecular mass = 2 x Vapour density = 2 x 44 = 88
n = (Molecular mass / Empirical
Formula mass ) = 88 / 44 = 2
Molecular formula = Empirical
formula x n
C2 H4 O x 2
C4 H8 O2
2.A compound on
analysis gave the following percentage composition: Na=14.31% S = 9.97%, H =
6.22%, O = 69.5%, calcualte the molecular formula of the compound on the
assumption that all the hydrogen in the compound is present in combination with
oxygen as water of crystallisation. Molecular mass of the compound is 322 [Na =
23, S = 32, H = 1, 0 = 16].
Solution :-
Calculation of empirical formula
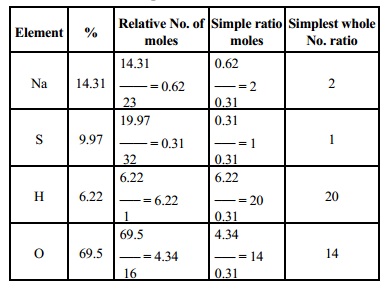
The empirical formula is Na2 SH20O14
Calculation of Molecular formula
Empirical formula mass = (23 x 2)
+ 32 + (20 x 1) + (16 x 14) = 322
n = (Molecular mass / Empirical formula mass ) =
322 / 322 = 1
Hence molecular formula = Na2 SH20 O14
Since all hydrogens are present as H2O
in the compound, it means 20 hydrogen atoms must have combined. It means 20
hydrogen atoms must have combined with 10 atoms of oxygen to form 10 molecules
of water of crystallisation. The remaining (14 - 10 = 4) atoms of oxygen should
be present with the rest of the compound.
Hence, molecular formula = Na2SO4.10H2O.
Problems for Practice
1. An organic compound was found to have
contained carbon = 40.65%, hydrogen = 8.55% and Nitrogen = 23.7%. Its vapour -
density was found to be 29.5. What is the molecular formula of the compound?
Ans:- C2H5NO
2. A compound contains 32% carbon, 4% hydrogen
and rest oxygen. Its vapour density is 75. Calculate the empirical and
molecular formula. Ans:- C2H3O3, C4H6O6
An acid of molecular mass 104 contains 34.6%
carbon, 3.85% hydrogen and the rest is oxygen. Calcualte the molecualr formula
of the acid.
What is the simplest formula of the compound
which has the following percentage composition: carbon 80%, Hydrogen 20%, If
the molecular mass is 30, calcualte its molecular formula.
Related Topics