Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Defects of Rutherford's model
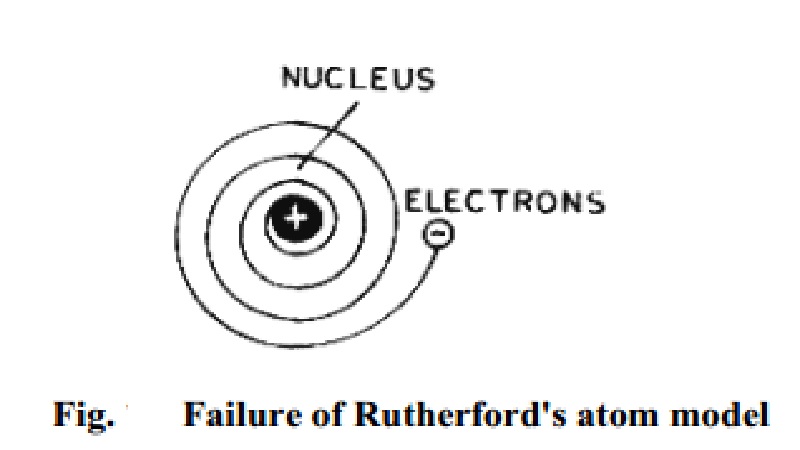
Defects of Rutherford's
model
According
to Rutherford's model, an atom consists of a positive nucleus with the
electrons moving around it in circular orbits. However it had been shown by J.
C. Maxwell that whenever an electron is subjected to acceleration, it emits
radiation and loses energy. As a result of this, its orbit should become
smaller and smaller Fig.. and finally it should drop into the nucleus by
following a spiral path. This means that atom would collapse and thus
Rutherford's model failed to explain stability of atoms.
Another drawback of the Rutherford's model is
that it says nothing about the electronic structure of the atoms i.e., how the
electrons are distributed around the nucleus and what are the energies of these
electrons. Therefore, this model failed to explain the existence of certain
definite lines in the hydrogen spectrum.
Postulates of Bohr's model of an atom
Rutherford's Nuclear
model of a atom
1.
An atom consists of a tiny positively charged
nucleus at its centre.
2.
The positive charge of the nucleus is due to
protons. The mass of the nucleus, on the other hand, is due to protons and some
neutral particles each having mass nearly equal to the mass of proton. This
neutral particle, called neutron, was discovered later on by Chadwick in 1932.
Protons and neutrons present in the nucleus are collectively also known as
nucleons. The total number of nucleons is termed as mass number(A) of the atom.
3.
The nucleus is surrounded by electrons that move
around the nucleus with very high speed in circular paths called orbits. Thus, Rutherford's model of
atom resembles the solar system in which the sun plays the role of the nucleus
and the planets that of revolving electrons.
4.
The number of electrons in an atom is equal to
the number of protons in it. Thus, the total positive charge of the nucleus
exactly balances the total negative charge in the atom making it electrically
neutral. The number of protons in an atom is called its atomic number(Z).
5.
Electrons and the nucleus are held together by
electrostatic forces of attraction.
Related Topics