Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Metallurgy - Purification of metals
Metallurgy - Purification of metals
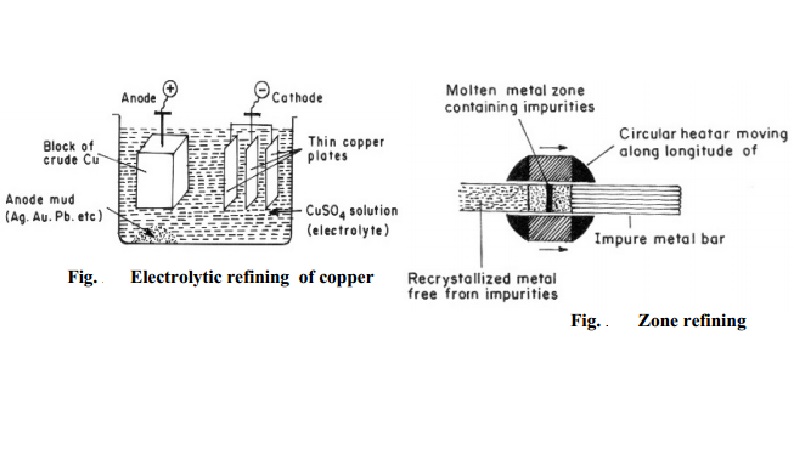
(a) Electrolytic refining
This is
one of the most convenient and important method of refining and gives a metal
of high purity. This method is applicable to many metals such as Cu, Ag, Pb, Au, Ni, Sn, Zn etc. The
blocks of impure metal form the anode and thin sheets of pure metal form the
cathode. A solution of a salt of the metal is taken as an electrolyte. On
passing an electric current through the solution pure metal dissolves from the
anode and deposits on cathode. By this process, more metal ions undergo
reduction and pure metal is deposited at the cathode. The insoluble impurities
either dissolve in the electrolyte or fall at the bottom and collect as anode mud. For example, in the refining
of copper, impurities like Fe and Zn dissolve in the electrolyte, while Au, Ag
and Pt are left behind as anode mud.
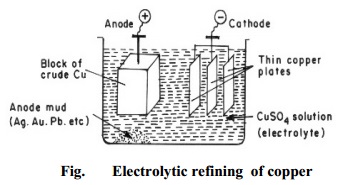
Copper: During the electrolytic refining of a copper, a thick block of impure copper is made anode, and thin plate of pure
copper is made cathode. Copper sulphate solution is used as an electrolyte. On
passing electric current, following reactions take place:
Cu2+ ions (from copper sulphate solution) go to the cathode
(negative electrode), where they are reduced to copper, which gets deposited on
the cathode.
Cu2+ + 2e-
- > Cu
Copper (of impure anode) forms copper ions, and these go into solution
of electrolyte.
Cu
- > Cu2+ + 2e-
Thus, the net result is transfer of pure copper from anode to the
cathode. Impurities like zinc, iron, etc., go into solution; while noble impurities
like silver, gold, etc., are left behind as anode mud. Copper is refined to
99.98% pure copper by electrolytic refining (Fig.).
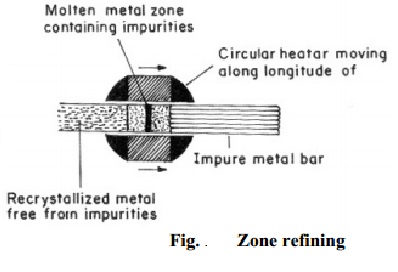
(b) Zone refining
This method is employed for preparing highly pure metal (such as
silicon, tellurium, germanium), which are used as semiconductors. It is based
on the principle that melting point of a substance is lowered by the presence
of impurities. Consequently, when an impure molten metal is cooled, crystals of
the pure metal are solidified, and the impurities remain behind the remaining
metal. (Fig.).
The
process consists in casting the impure metal in the form of a bar. A circular
heater fitted around this bar is slowly moved longitudinally from one end to
the other. At the heated zone, the bar melts, and as the heater moves on, pure
metal crystallizes, while the impurities pass into the adjacent molten part. In
this way, the impurities are swept from one end of the bar to the other. By
repeating the process, ultra pure metal can be obtained.
(c) Mond's process
Thermal methods include methods as carbonyl method, decomposition of
hydrides etc. The carbonyl method is used for the refining of metals like Ni
and Fe. For example, in case of nickel, the impure metal is heated with CO. The
nickel carbonyl thus formed is then decomposed (after distilling off the
impurities) to get pure nickel metal and CO. The process is known as Mond's process.
Ni + 4CO - > Ni(CO)4 - > Ni + 4CO
It is
based on the following facts:
1. Only nickel (and not Cu, Fe, etc.) forms a volatile carbonyl, Ni(CO)4,
when CO is passed over it at 500C.
2.
the nickel carbonyl decomposes at 1800C
to yield pure nickel.
Related Topics