Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Postulates of Bohr's model of an atom
Defects of Rutherford's
model
According
to Rutherford's model, an atom consists of a positive nucleus with the
electrons moving around it in circular orbits. However it had been shown by J.
C. Maxwell that whenever an electron is subjected to acceleration, it emits
radiation and loses energy. As a result of this, its orbit should become
smaller and smaller Fig.. and finally it should drop into the nucleus by
following a spiral path. This means that atom would collapse and thus
Rutherford's model failed to explain stability of atoms.
Another drawback of the Rutherford's model is
that it says nothing about the electronic structure of the atoms i.e., how the
electrons are distributed around the nucleus and what are the energies of these
electrons. Therefore, this model failed to explain the existence of certain
definite lines in the hydrogen spectrum.
Postulates of Bohr's model of an atom
To overcome the above defects of Rutherford's
model, Niels Bohr in 1913 gave a modification based on Quantum theory of
radiation. The important postulates are:
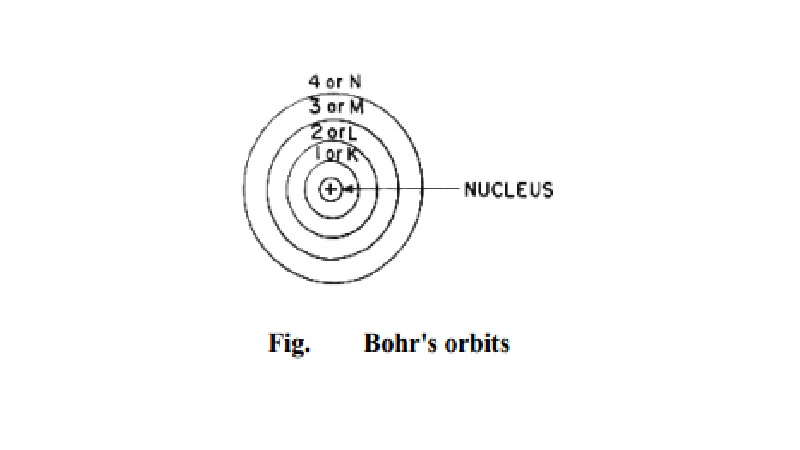
1.
The electrons revolve round the nucleus only in
certain selected circular paths called orbits. These orbits are associated with
definite energies and are called energy shells or energy levels or quantum
levels. These are numbered as 1, 2, 3, 4 ….. etc. (starting from the nucleus)
are designated as K, L, M, N ….etc. (Fig.).
2.
As long as an electron remains in a particular
orbit, it does not lose or gain energy. This means that energy of an electron
in a particular path remains constant. Therefore, these orbits are also called
stationary states.
3.
Only those orbits are permitted in which angular
momentum of the electron is a whole number multiple of h/2pi , where h is the
Planck 's constant. An electron moving in a circular orbit has an angular
momentum equal to mnr where m
is the mass of the electron and n, the angular momentum, mvr is a
whole number multiple of h/2pi i.e.,
mvr = nh/2pi, where n = 1,2,3…
In other words, angular velocity of electrons in an atom is quantised.
4.
If an electron jumps from one stationary state
to another, it will absorb or emit radiation of a definite frequency giving a
spectral line of that frequency which depends upon the initial and final
levels. When an electron jumps back to the lower energy level, it radiates same
amount of energy in the form of radiation.
Limitation of Bohr's Theory
1)
According to Bohr, the radiation results when an
electron jumps from one energy orbit to another energy orbit, but how this
radiation occurs is not explained by Bohr.
2)
Bohr Theory had explained the existence of
various lines in H-spectrum, but it predicted that only a series of lines
exist. At that time this was exactly what had been observed. However, as better
instruments and techniques were developed, it was realized that the spectral
line that had been thought to be a single line was actually a collection of
several lines very close together (known as fine spectrum). Thus for example,
the single H¥-spectral
line of Balmer series consists of many lines very close to each other.
3)
Thus the appearance of the several lines implies
that there are several energy levels, which are close together for each quantum
number n. This would require the existence of new quantum numbers.
4)
Bohr's theory has successfully explained the
observed spectra for hydrogen atom and hydrogen like ions (e.g. He+,
Li2+, Be3+ etc.), it can not explain the spectral series
for the atoms having a large number of electrons.
5)
There was no satisfactory justification for the
assumption that the electron can rotate only in those orbits in which the
angular momentum of the electron (mvr ) is a
whole number multiple of h/2pi, i.e. he could not
give any explanation for using the principle of quantisation of angular
momentum and it was introduced by him arbitrarily.
6)
Bohr assumes that an electron in an atom is
located at a definite distance from the nucleus and is revolving round it with
definite velocity, i.e. it is associated with a fixed value of momentum. This
is against the Heisenberg's Uncertainty Principle according to which it is
impossible to determine simultaneously with certainty the position and the
momentum of a particle.
7)
No explanation for Zeeman effect: If a substance
which gives a line emission spectrum, is placed in a magnetic field, the lines
of the spectrum get split up into a number of closely spaced lines. This
phenomenon is known as Zeeman effect. Bohr's theory has no explanation for this
effect.
8)
No explanation of the Stark effect: If a
substance which gives a line emission spectrum is placed in an external
electric field, its lines get spilt into a number of closely spaced lines. This
phenomenon is known as Stark effect. Bohr's theory is not able to explain this
observation as well.
Related Topics