Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Bragg's Equation and spectrometer method
X-RAYS AND CRYSTAL STRUCTURE
X-rays are electromagnetic waves of very short wavelength.
The wave nature of X-rays is not confirmed by diffraction experiment,
because a grating of about 40 million
ruling per cm is required for diffraction experiment. The preparation of such a grating is highly impossible. At the same time,
crystallographers believed that atoms in
crystals are regularly arranged with an interatomic distance of about 10-8 cm. The wavelength of
X-rays is, also, in the order of 10-8 cm. Based
on this, Laue suggested that crystal can be used as a three
dimensional diffraction grating for
X-rays. This suggestion is carried out successfully by Laue in his experiment using zinc sulphide crystal. The photograph
obtained is known as Laue diffraction
pattern.
The Laue experiment confirmed
the wave nature of X-rays and also established
the fact that atoms or ions in crystals are arranged in regular three dimensional lattice. Though Laue diffraction pattern
gives more information about the symmetry
of crystals, the interpretation of the pattern seems to be difficult.
Bragg's Equation
W.L.Bragg and W.H.Bragg derived a mathematical relation
to determine interatomic distances from X-ray diffraction patterns. The
scattering of X-rays by crystals could
be considered as reflection from successive planes of atoms in the crystals. However, unlike reflection of ordinary
light, the reflection of X-rays can take place only at certain angles which are
determined by the wavelength of the
X-rays and the distance between the planes in the crystal. The fundamental equation which gives a simple relation between the
wavelength of the X-rays, the
interplanar distance in the crystal and the angle of reflection, is known as Bragg's equation.
Bragg's equation is nl = 2d sinq
where n is the
order of reflection
l is the wavelength of X-rays
d is the
interplanar distance in the crystal
q is the angle of reflection
Significance of Bragg's equation
1) If we use X-rays of known wavelength (l), then the interatomic distance (d)
in an unknown crystal can be calculated. On the other hand, if we use a crystal whose interatomic distance 'd' is known, then the
wavelength of
X-rays can be calculated.
2. The Bragg's equation gives the essential condition
for diffraction to occur.
3. When the
experiment is done, there will be a maximum reflection at a
particular angle q. That angle is
noted. It corresponds to first order reflection (n=1).
If the angle 'q' is increased,
maximum reflection occurs at some other higher angle. It corresponds to second order
reflection (n=2). Similarly, third, fourth and higher order of reflection occur at
certain specific angles. The values of
angles obtained are in accordance with the Bragg's equation. Hence Bragg's equation is experimentally verified.
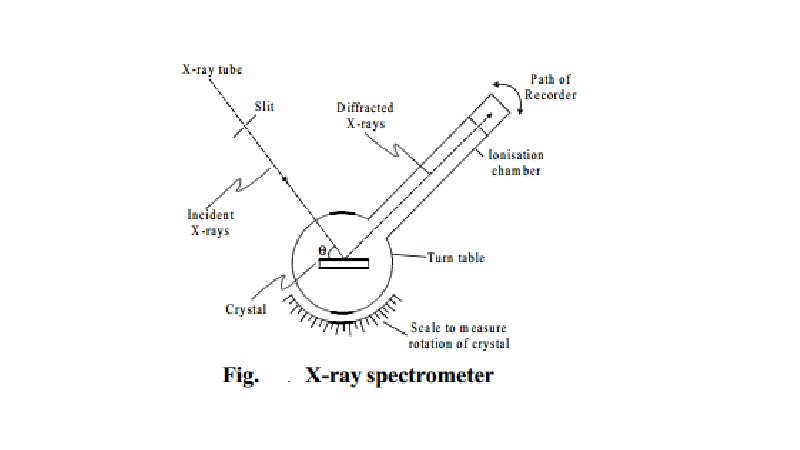
Bragg's spectrometer method
This method is one of the
important method for studying crystals using X-rays. The apparatus consists of a X-ray tube from which a narrow beam
of X-rays is allowed to fall on the crystal mounted on a
rotating table.The rotating table is
provided with scale and vernier, from which the angle of incidence, q can be measured.
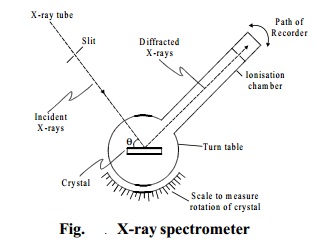
An arm which is rotating about the same axis as the
crystal table, carries an ionisation
chamber. The rays reflected from the crystal enter into the ionisation chamber and ionise the gas present inside. Due to the
ionisation, current is produced which is
measured by electrometer. The current of ionisation is a direct measure of intensity of reflected beam from the
crystal. For different angles of incidence, the corresponding ionisation
current is measured from the electrometer.
These values are plotted in the form of graph.
For sodium chloride, the maximum reflection or peaks for
100 plane occurred at q = 5.9 o , 11.85 o and 18.15 o . The sines of these angles are
0.103, 0.205 and 0.312 which are in
the ratio 1:2:3. These peaks represent the first,second and third order reflections. The ratio confirms the
correctness of Bragg's equation.
Related Topics