Chapter: Biochemistry: The Three-Dimensional Structure of Proteins
The Collagen Triple Helix
The Collagen Triple Helix
Collagen, a component of bone and connective tissue, is the most abundant protein in vertebrates. It is organized in water-insoluble fibers of great strength.
A collagen fiber consists of three polypeptide chains wrapped around each other in a ropelike twist, or triple helix. Each of the three chains has, within limits, a repeating sequence of three amino acid residues, X-Pro-Gly or X-Hyp-Gly, where Hyp stands for hydroxyproline, and any amino acid can occupy the first position, designated by X.
Proline and hydroxyproline can constitute up to 30% of the residues in collagen. Hydroxyproline is formed from proline by a specific hydroxylating enzyme after the amino acids are linked together. Hydroxylysine also occurs in collagen. In the amino acid sequence of collagen, every third position must be occupied by glycine. The triple helix is arranged so that every third residue on each chain is inside the helix. Only glycine is small enough to fit into the space available (Figure 4.11).
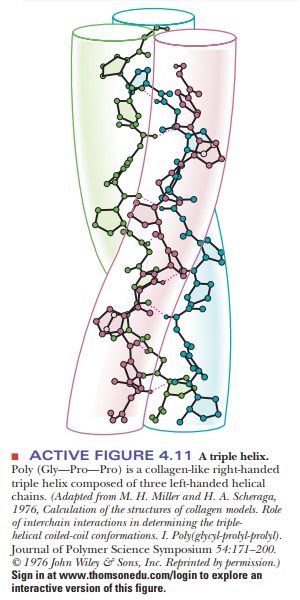
The three individual collagen chains are themselves helices that differ from the α-helix. They are twisted around each other in a superhelical arrangement toform a stiff rod. This triple helical molecule is called tropocollagen; it is 300 nm (3000 Å) long and 1.5 nm (15 Å) in diameter. The three strands are held together by hydrogen bonds involving the hydroxyproline and hydroxylysine residues.
The molecular weight of the triple-stranded array is about 300,000; each strand contains about 800 amino acid residues. Collagen is both intramolecularly and intermolecularly linked by covalent bonds formed by reactions of lysine and histidine residues. The amount of cross-linking in a tissue increases with age. That is why meat from older animals is tougher than meat from younger animals.
Collagen in which the proline is not hydroxylated to hydroxyproline to the usual extent is less stable than normal collagen. Symptoms of scurvy, such as bleeding gums and skin discoloration, are the results of fragile collagen. The enzyme that hydroxylates proline and thus maintains the normal state of col-lagen requires ascorbic acid (vitamin C) to remain active. Scurvy is ultimately caused by a dietary deficiency of vitamin C.
Related Topics