Chapter: Biochemistry: The Three-Dimensional Structure of Proteins
Secondary Structure of Proteins: Irregularities in Regular Structures
Irregularities in Regular Structures
Other helical structures are found in proteins. These are often found in shorter stretches than with the α-helix, and they sometimes break up the regular nature of the α-helix. The most common is the 310 helix, which has three residues per turn and 10 atoms in the ring formed by making the hydrogen bond. Other common helices are designated 27 and 4.416, following the same nomenclature as the 310 helix.
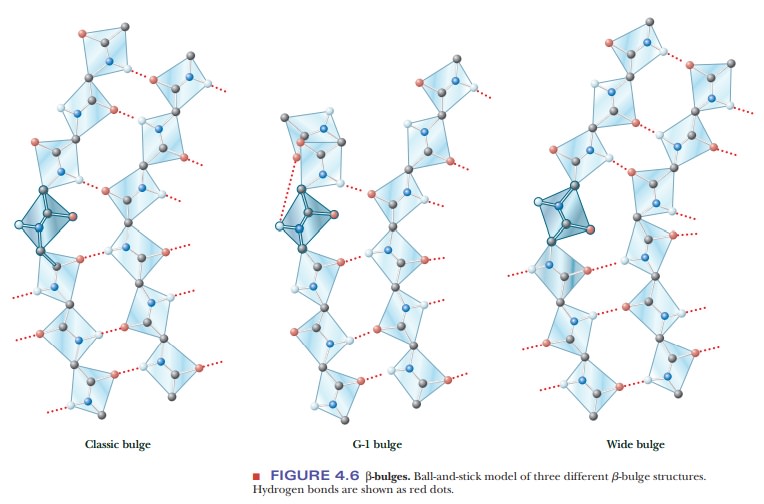
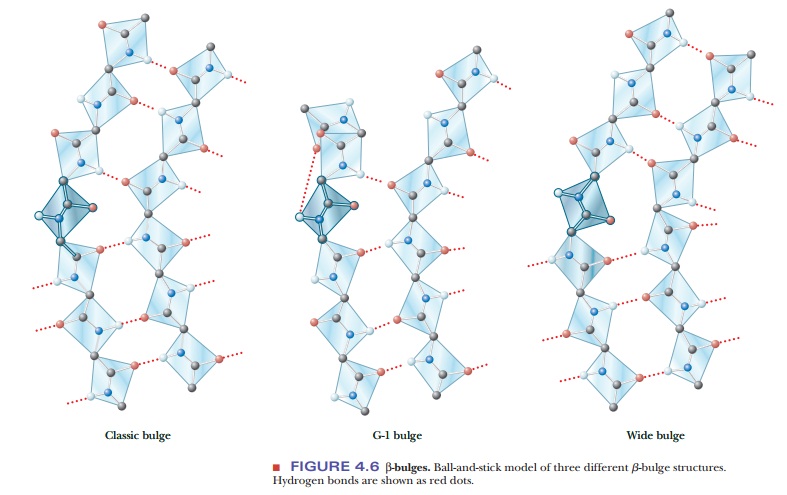
A β-bulge is a common nonrepetitive irregularity found in antiparallel β-sheets. It occurs between two normal β-structure hydrogen bonds and involves two residues on one strand and one on the other. Figure 4.6 shows typical β-bulges.
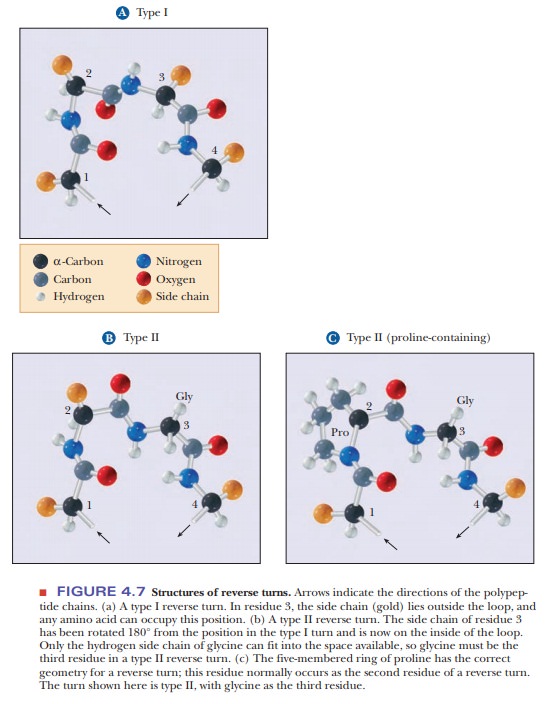
Protein folding requires that the peptide backbones and the secondary structures be able to change directions. Often a reverse turn marks a transition between one secondary structure and another. For steric (spatial) reasons, gly-cine is frequently encountered in reverse turns, at which the polypeptide chain changes direction; the single hydrogen of the side chain prevents crowding (Figures 4.7a and 4.7b). Because the cyclic structure of proline has the correct geometry for a reverse turn, this amino acid is also frequently encountered in such turns (Figure 4.7c).
Related Topics