Chapter: Biochemistry: The Three-Dimensional Structure of Proteins
Hydrophobic Interactions:A Case Study in Thermodynamics
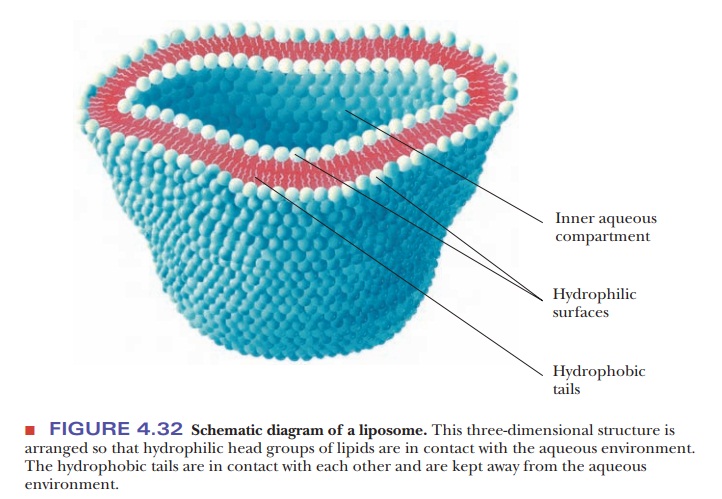
Hydrophobic Interactions:A Case Study in Thermodynamics
Hydrophobic interactions have important consequences in biochemistry and play a major role in protein folding. Large arrays of molecules can take on definite structures as a result of hydrophobic interactions. We have already seen the way in which phospholipid bilayers can form one such array. Recall that phospholipids are molecules that have polar head groups and long nonpolar tails of hydrocarbon chains. These bilayers are less complex than a folded protein, but the interactions that lead to their formation also play a vital role in protein folding. Under suitable conditions, a double-layer arrangement is formed so that the polar head groups of many molecules face the aqueous environment, while the nonpolar tails are in contact with each other and are kept away from the aqueous environment. These bilayers form three-dimensional structures called liposomes (Figure 4.32). Such structures are useful model systems for biological membranes, which consist of similar bilayers with proteins embedded in them. The interactions between the bilayer and the embedded proteins are also examples of hydrophobic interactions. The very existence of membranes depends on hydrophobic interactions. The same hydrophobic interactions play a crucial role in protein folding.
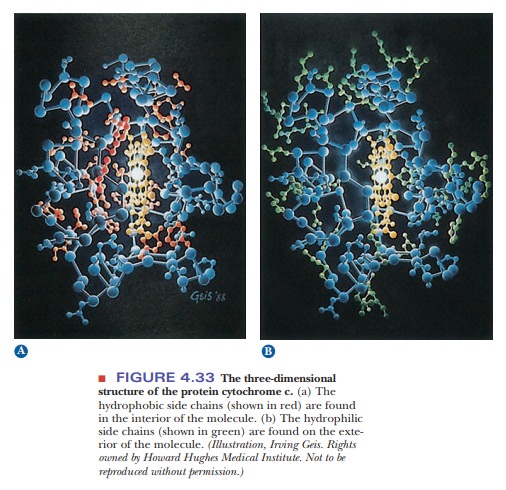
Hydrophobic interactions are a major factor in the folding of proteins into the specific three-dimensional structures required for their functioning as enzymes, oxygen carriers, or structural elements. It is known experimentally that proteins tend to be folded so that the nonpolar hydrophobic side chains are sequestered from water in the interior of the protein, while the polar hydro-philic side chains lie on the exterior of the molecule and are accessible to the aqueous environment (Figure 4.33).
What makes hydrophobic interactions favorable?
Hydrophobic interactions are spontaneous processes. The entropy of the Universe increases when hydrophobic interactions occur.
∆Suniv > 0
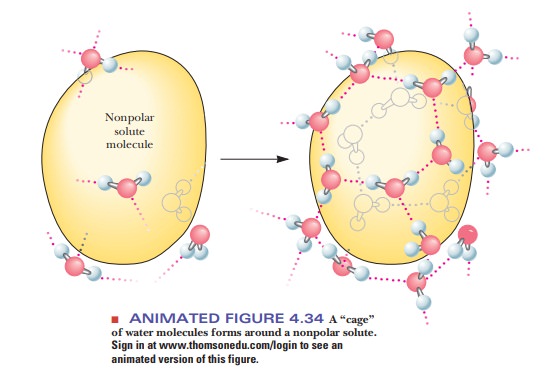
As an example, let us assume that we have tried to mix the liquid hydrocarbon hexane (C6H14) with water and have obtained not a solution but a two-layer system, one layer of hexane and one of water. Formation of a mixed solution is nonspontaneous, and the formation of two layers is spontaneous. Unfavorable entropy terms enter into the picture if solution formation requires the creation of ordered arrays of solvent, in this case water (Figure 4.34). The water molecules surrounding the nonpolar molecules can hydrogen bond with each other, but they have fewer possible orientations than if they were surrounded by other water molecules on all sides. This introduces a higher degree of order, preventing the dispersion of energy, more like the lattice of ice than liquid water, and thus a lower entropy. The required entropy decrease is too large for the process to take place. Therefore, nonpolar substances do not dissolve in water; rather, nonpolar molecules associate with one another by hydrophobic interactions and are excluded from water.
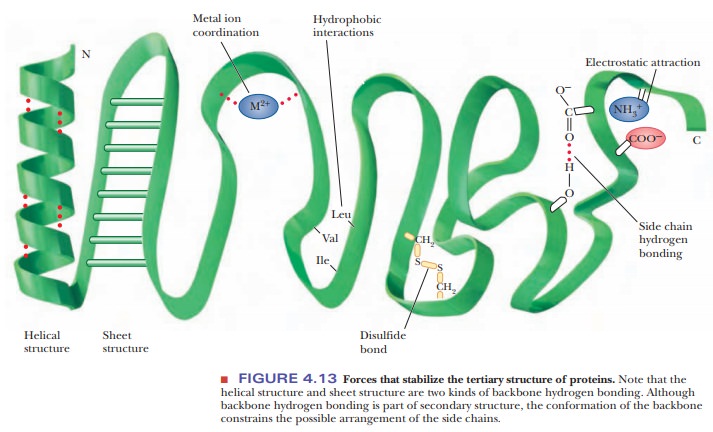
Many people think of hydrophobic interactions between amino acids back-ward. For example, if we look at Figure 4.13 and see the indication of hydro-phobic interactions between leucine, valine, and isoleucine, we might conclude that hydrophobic interactions refer to an attraction for these amino acids for each other. However, we now know that in reality it is not so much the attrac-tion of the nonpolar amino acids for each other, but rather it is more that they are forced together so that water can avoid having to interact with them.
Related Topics