Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Temperature Dependance Of Rate Constant
TEMPERATURE DEPENDANCE OF
RATE CONSTANT
It is a common observation that rates of
reactions increase with increase in temperature of the reaction mixture.
Keeping the concentration of the reactants constant, the rate is found to be
two times its initial value, when measured at a temperature 10 K greater than
the initial temperature. However, the exact value of the rate constant
determined at various temperature is predicted by using Arrhenius equation.
This expression is obeyed by most of the reactions. Arrhenius equation is given
as
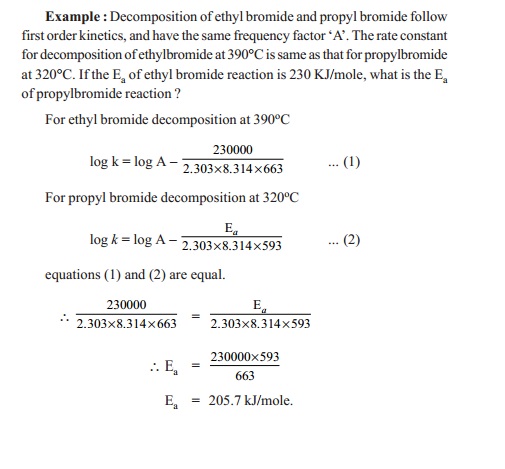
k = A e-Ea/RT
where k
= rate constant, Ea = activation energy, A = frequency factor, R =
gas constant, T = temperature in Kelvin. If k1
and k2 are the rate constants measured at two different T1
and T² temperatures respectively, then Ea can be
determined as follows :
Arrhenius equation for two different
temperatures T1 and T2 are :
log k1
= log A - (Ea / 2. 303R T1)
log k2
= log A - (Ea / 2. 303R T2)
where k1
and k2 are the rate
constants at temperature T1 and T2 respectively.
log k2 - log k1= - (Ea / 2. 303R T2) + (Ea / 2. 303R T1)
log(k2 /k2) = Ea /
2. 303R ( T2-T1 /
T1T2)
If R =
1.987 cals.mol-1 , then unit of E is 10-3 k.cal.
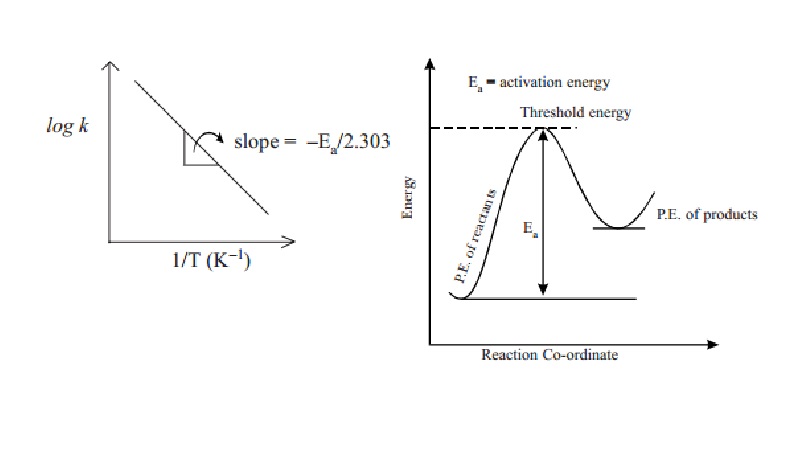
Also, a plot of log k against 1/T values gives a straight line with slope value equals
to -E a/2.303 R and intercept value equals to log A. When Ea
is a positive value, and if T2 > T1 then k2 > k1. That is, rate constant value at higher temperature
is greater than rate constant value at lower temperature. Under such
conditions, plot of log k against 1/T gives a negative slope straight line.
From the slope of the straight line, Ea can be calculated.
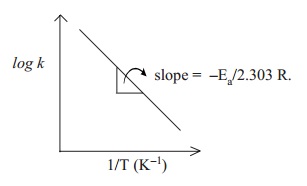
Reactant molecules come into contact through
collisions for transformation into product molecules. Since, not all collisions
are successful in producing the product molecules, all colliding molecules must
possess certain minimum energy called as the threshold energy which is needed to make the collisions effective
and successful. The additional energy required by the molecules to attain the
threshold energy in addition to the energy of colliding molecules is called as
activation energy 'Ea'. Thus, activation
energy = threshold energy - Energy of
colliding molecules. Generally, this
Ea value is higher than the potential energies of reactants and
products. Thus, Ea is considered as an energy barrier that must be
crossed by the reactant molecules before getting converted to actual product
molecules.
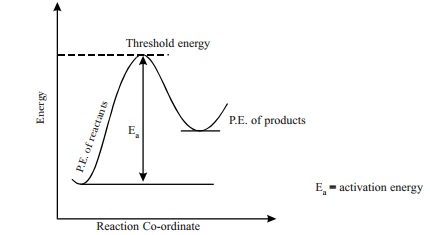
Potential energy diagram of a reaction. The
molecular state present at the Ea position in the potential energy diagram, is
considered as the intermediate product (or) the transition state. Thus Ea, is
also the energy required to form the activated state or the intermediate, which
is necessary to form the products. Ea is a characteristic value of a reaction.
The rate, rate constant, and their temperature dependance are determined by the
value of Ea. Higher the value of Ea, slower is the rate of the reaction.
Related Topics