Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Determination of rate constant of acid catalysed hydrolysis of an ester
Determination of rate
constant of acid catalysed hydrolysis of an ester
The hydrolysis reaction of an ester in pure
water is a slow reaction and when a mineral acid like hydrochloric acid is
added, the rate of the reaction is enhanced since the H+ ions from
the mineral acid acts as the catalyst. The acid catalysed hydrolysis of an
ester follows pseudo first order kinetics. The reaction can be represented as :
CH3COOCH3 + H2O
-- H+ -- > CH3COOH
+ CH3OH
Methyl acetate
The overall rate of the reaction depends on the
concentrations of reactants and also on the catalyst concentration.
Rate = k3
[ester] [H2O] [H+]
k3 = rate constant of the third order reaction.
Therefore the true order of the reaction is
3.0. Since water is used as the solvent, its concentration is excess.
Weight of 1 lit (1000 cc) of water = 1000 gm =
1 kg (\ density
of water = 1 gm/cc)
No. of moles of water in 1 lit = 1000 / 18
Concentration of pure water = 55.55 moles
If 1 molar aqueous solution
of ester is used then, 1 mole of water will be consumed for its complete
hydrolysis. After the completion of hydrolysis, 55.55 - 1.0 = 54.55 moles water
will be present in the medium. Therefore change in the concentration of water
considered as negligible and concentration of water is assumed to be constant.
Since acid acts the catalyst, there will be, no change in the catalyst concentration
before initial and after the completion periods of times of the reactions.
Hence [H+] is considered as a constant value. Hence the expression
can be rewritten as
Rate = k3'
[ester] where k3' = pseudo
first order rate constant = k3 [H+] [H2O]. In
this rate expression rate of the reaction is directly proportional to ester concentration only.
Procedure
Initially to a definite volume of (100 ml)
hydrochloric acid (0.5 N), 10 ml of ester is added and the start of the
reaction corresponds to time of addition of ester. The rate of the reaction is
followed by withdrawing a definite volume of the reaction mixture consisting of
the ester and acid at various time intervals and arresting the further progress
of reaction by adding ice. The whole cold mixture is titrated with standard
NaOH (0.1 N) using phenolphthalein as the indicator.
Let the volume of alkali consumed at t = 0 be Vo cc which is
equivalent to the amount of hydrochloric acid present in the definite volume of
the reaction mixture drawn out at regular intervals of time. If Vt
cc is the volume of alkali consumed for the same definite volume of the
reaction mixture drawn out after reaction time ' t', then (V t - Vo) cc is equivalent to the
acetic acid produced by the hydrolysis of ester in time ' t'. A final titration is done after about 8 hours or after
refluxing the solution for 45 mins to complete the hydrolysis which is V¥ cc. (V¥- V o)
cc is equivalent to acetic acid produced from complete hydrolysis of ester.
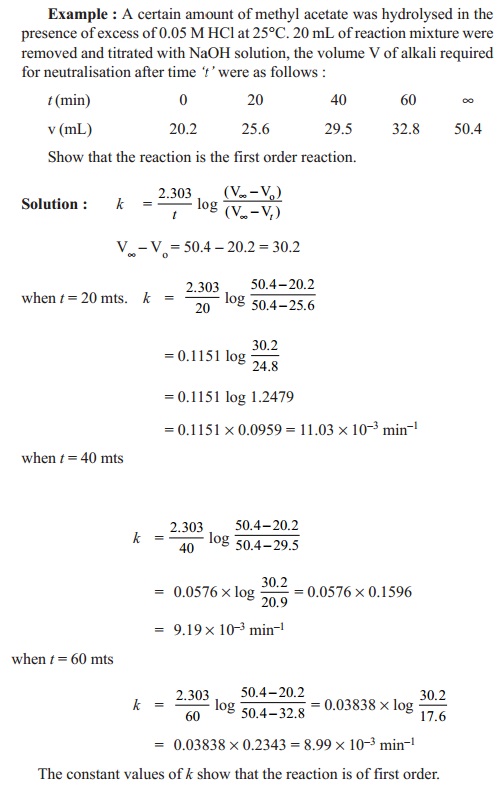
Calculations
The initial concentration of ester = a a (V¥- V o) cc Concentration of ester
reacted at 't' = x a (Vt - V o)
cc
Concentration of ester remaining at time 't' = (a - x) a (V¥- V t)
A / (a-x) = ((V¥- V o)) / ((V¥- V t))
The
first order rate expression for the hydrolysis of ester can be
written
as
k
= (2.303/t ) log ((V¥- V o)) / ((V¥- V t))
Related Topics