Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Simple And Complex Reactions : Difference and Types

Simple
And Complex Reactions
SIMPLE
AND COMPLEX REACTIONS
A simple reaction takes place in a single step.
Simple reactions are also known as elementary reactions. One step reactions are
elementary reactions. In some reactions many side reactions occur along with
the main reaction involving product formation.
Reactions which do not take place in a single
step but take place in a sequence of a number of elementary steps are called as
complex reactions.
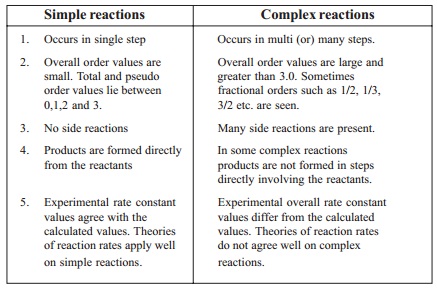
1.Simple reactions
i.
Occurs in single step
ii.
Overall order values are small. Total
and pseudo order values lie between 0,1,2 and 3.
iii.
No side reactions
iv.
Products are formed directly from the
reactants
v.
Experimental rate constant values agree
with the calculated values. Theories of reaction rates apply well on simple
reactions.
2. Complex reactions
i.
Occurs in multi (or) many steps.
ii.
Overall order values are large and
greater than 3.0. Sometimes fractional orders such as 1/2, 1/3, 3/2 etc. are
seen.
iii.
Many side reactions are present.
iv.
In some complex reactions
v.
products are not formed in steps
directly involving the reactants.
vi.
Experimental overall rate constant
values differ from the calculated values. Theories of reaction rates do not
agree well on complex reactions.
Types of Complex reaction
The reactions in which the reactant forms an
intermediate and the intermediate forms the product in one or many subsequent
reactions are called as consecutive or sequential reactions. In such reactions
the product is not formed directly from the reactant. Various steps in the
consecutive reaction are shown as below :
A --- k1--- > B --- k1
--- > C
A = reactant ; B = intermediate ; C = product.
Initially only the reactant A will be present. As the reaction starts, A
produces an intermediate B through k1
rate constant. As and when B is formed, it produces the product C through k2 rate constant. After the
completion of reaction only 'C' is present and concentrations of A and B will
be zero.
Example of consecutive
reactions
Saponification of a diester in presence of an
alkali :
R'OOC- (CH 2)n-COOR ---k1
--- > R'OOC-(CH 2)n-COOH -- -- k2 --- > HOOC -
(CH 2)n - COOH
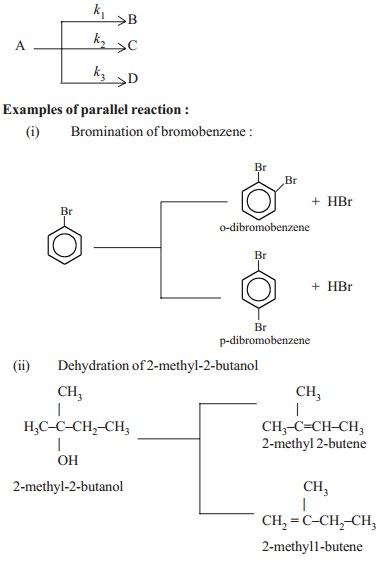
(ii) Parallel reactions
In these group of reactions, one or more
reactants react simultaneously in two or more pathways to give two or more
products. The parallel reactions are also called as side reactions.
The reactant A reacts to give products B,C,D
separately by following three different reaction pathways involving different k1, k2, k3
rate constants respectively. Among the many side reactions, the reaction in
which maximum yield of the product obtained is called as the main or major
reaction while the other reactions are called as side or parallel reactions.
Examples of parallel reaction
:
Bromination of bromobenzene :
(iii) Opposing reactions
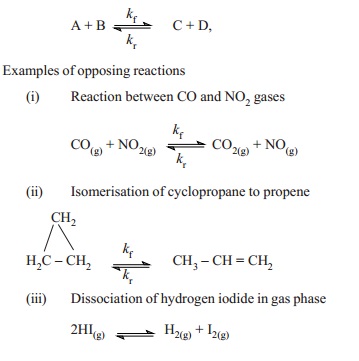
In opposing reactions the products formed react
back simultaneously to form the reactants. These reactions are also called as
reversible reactions.
A + B --
Kf-- > < --Kr
---C + D
Examples of opposing reactions
(i) Reaction between CO and NO2
gases
(ii) Isomerisation of cyclopropane to propene
(iii) Dissociation of hydrogen iodide in gas
phase
Related Topics