Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Structures of interhalogen compounds
INTERHALOGEN COMPOUNDS OR INTERHALOGENS
Each halogen combines with another halogen to form
several compounds known as interhalogen compounds. The less electronegative
element is written first. In naming also, the less electronegative element is
mentioned first.
They are divided into four types.
AX : CIF , BrF
,BrCl, ICl, IBr.
AX3: ClF3 BrF3 ICl3
AX5 : BrF5 IF5
AX7 : IF7
They can all be prepared by direct combination or by the
action of a halogen on a lower
interhalogen, the product formed depends on the conditions.
473K
Cl2+ F2 (equal volume) ----- --- > 2ClF
(AX type)
I2 + Cl2 liquid (equi molar) ®
2ICl (AX type)
573K
Cl2 + 3F2 (excess)
---- --- > 2 ClF3 (AX3 type)
Br2 + 3F2 (diluted with nitrogen)® 2Br F3 Br2 + 5F2 (excess) ® 2Br F5 (AX5 Type)
I2 + 5F2 (Excess) ® 2IF5 (AX5 Type)
IF5 + F2 (Excess)
------- 573K ----- --- > IF7 (AX7 Type)
The bonds are essentially covalent because of the small
electronegativity difference, and
the melting and boiling points increase as the difference in electronegativity increases.
The interhalogens are generally more reactive than the halogens (except F) because the A-X bond is weaker than the X-X bond in the halogens. The reactions are similar to those of the halogens. Hydrolysis gives halide and oxyhalide ions, the oxyhalide ion being formed from the larger halogen present.
BrF5 + 3 OH- ---
--- > 5F¯ + BrO3¯ + 3 H+
Bromate
ICl + OH- --- - ----------- --- > Cl ¯ + OI ¯ + H+
hypoiodite
Structures of interhalogen compounds
Interhalogen compounds are generally covalent compounds
in which the larger halogen forms the
central atom.
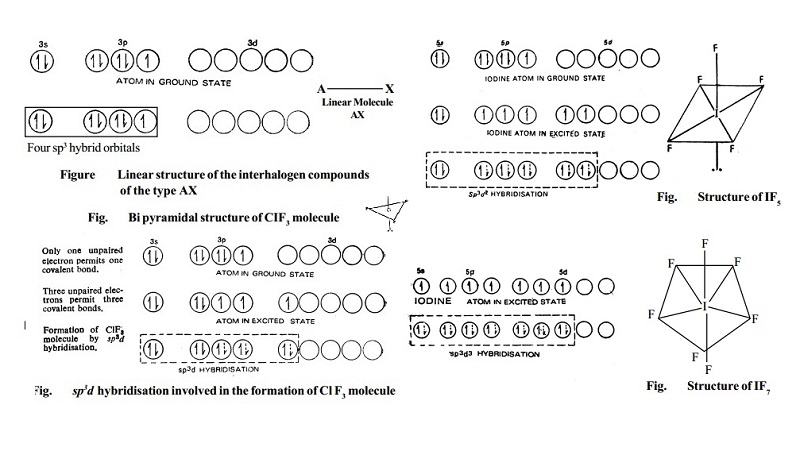
1. Type AX.
As excepted, the compounds of the type AX are linear. Thus CIF,
BrF, BrCl, IBr and ICI are all linear in structure.
Electronic structure of Chlorine atom, in the ground state and hybridised state is represented as in Fig.
Although the spatial arrangement of the four electron pairs (bp = 1 and lps = 3) round the central chlorine atom is tetrahedral, due to the presence of three lone pairs of electrons in three hybrid orbitals, the shape of AX molecule gets distorted and become linear.
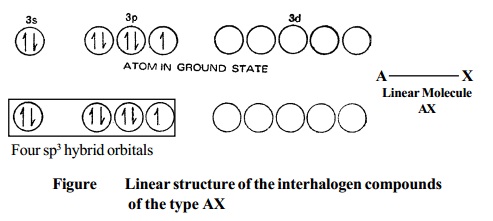
2. Type AX3 Compounds of the type AX3 have trigonal bipyramidal structure, Fig. for the ClF3 molecule.
Bipyramidal structure arises out of sp3d hybridisation involved in the formation of this compound, as illustrated in the Fig.. The three dotted arrows indicate electrons contributed by the three fluorine atoms (without lone pair it is T-shaped).
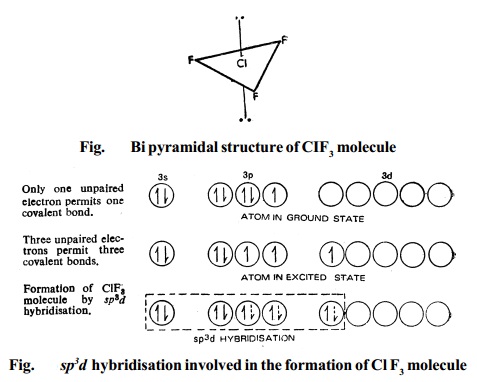
3. Type AX5 (IF5, BrF5, etc.) These
compounds are formed by sp3d2 hybridisation and hence have an octahedral
structure, as shown in Fig. for the formation of IF5 molecule (without lone pair it is square
pyramidal).
4. Type AX7 (IF7). This compound has
a pentagonalbipyramidal structure since
this is formed by sp3d3hybridisation.
Problem
An element A occupies group number 17 and period number 2, shows anomalous behaviour. A reacts with water forms a mixture of B, C and acid D. B and C are allotropes. A also reacts with hydrogen violently even in dark togive an acid D. Identify A,B,C and D. write the reactions.
Solution
i) The element A that occupies group number 17 and period number 2 is fluorine.
ii) Fluorine reacts with water and forms a mixture of
B and C
2F2 + 2H2O ® 4HF + O2
3F2 + 3H2O ® 6HF + O3
Therefore, B is Oxygen and C is Ozone.
iii) Fluorine reacts with hydrogen to give D.
F2 + H2 ® 2HF
D is
Hydrofluoric acid.
Related Topics