Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Isolation, Physical, Chemical Properties, Uses Of Fluorine
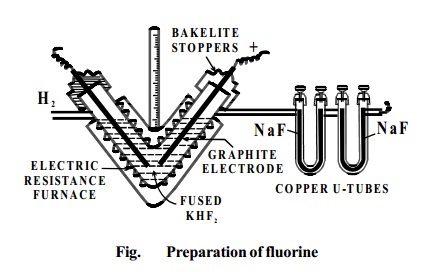
ISOLATION OF FLUORINE
Symbol - F Atomic number -9 Period Number :2
Valency -1 Atomic
mass-19 Group Number : 17
Fluorine does not occur free in nature. It occurs in the combined form.
Dennis' Method: This was devised by Dennis, Veeder and Rochow in 1931.
In this fluorine is prepared by the electrolysis of fused
sodium or potassium hydrogen fluoride
(perfectly dry) Electrolysis is carried out between graphite electrodes in a V-shaped electrically heated copper
tube. The ends of the tube are covered
with copper caps into which the graphite electrodes are fixed with bakelite cement. The copper tube is thickly lagged to
prevent loss of heat.
KHF2 ® KF + HF
HF ® H+ + F¯
2H+ + 2e- ® H
2 (At cathode)
2F
- - 2e- ® F2 (At anode)
Fluorine liberated at the anode is passed through the
U-tube containing
sodium fluoride. Thisremovesthe hydrogen fluoride
vapourscomingwithfluorine.
NaF
+HF ® NaHF2
Physical Properties
1. Fluorine is a gas and has pale greenish yellow
colour.
2. It has extremely pungent and penetrating odour. 3. It is heavier than air.
Chemical Properties
Fluorine is the most active member of halogen family.
1. Action with Hydrogen: Hydrogen explodes violently in fluorine even in the dark.
H2 + F2 ® 2HF
2. Action with non-metals: Non-metals like carbon, silicon and phosphorus burn in fluorine forming fluorides.
C + 2F2 ® CF4
Tetra fluoromethane
Si + 2F2 ® SiF4
Silicon tetrafluoride
2P + 5F2 ® 2PF5
Phosphorus pentafluoride
3. Action with metals: It reacts with metals forming corresponding
fluorides.
2Ag + F2 ® 2AgF
2Al + 3F2 ® 2AlF3
4. Formation of Interhalogen compounds: It forms a variety of inter halogen compounds with other halogens.
Br2 + 3F2 ® 2Br F3
I2 + 5F2 ® 2 IF5
Uses
1. Fluorine is used in the manufacture of a series of
compounds known as
freons. These non-toxic, non-combustible and volatile
liquids are used as refrigerants in
refrigerators, deep freezers and air conditioners. The most
common, freon is known as dichlorodifluoro methane CF2 Cl2.
2. CaF2 is used
as flux in metallurgy.
3. NaF is used as a preservative to prevent fermentation and also for preventing dental cavities.
4. SF6 is used
as an insulating material in high voltage equipment.
5. Teflon is used as container to store hydrofluoric
acid.
6. UF6 is used in the separation of U235 from U238.
Related Topics