Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Stability of orbitals
Stability of orbitals
According
to Hund's rule atoms having half-filled or completely-filled orbitals are
comparatively more stable and hence more energy is needed to remove an electron
from such atoms. The ionization potential or ionization enthalpy of such atom
is, therefore, relatively higher than expected normally from their position in
the periodic table.
The
extraordinary stability of half-filled and completely filled electron
configuration can be explained in terms of symmetry and exchange energy. The
half-filled and completely filled electron configurations have symmetrical
distribution of electrons and this symmetry leads to stability. Moreover, in
such configuration electron can exchange their positions among themselves to
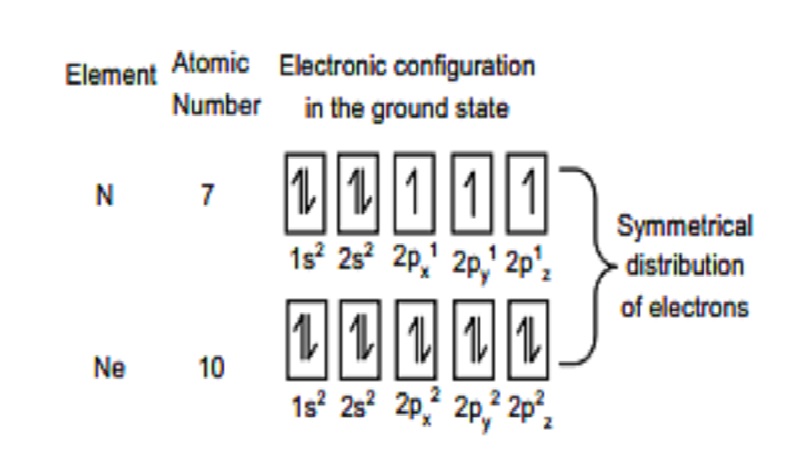
maximum extent. This exchange leads to stabilization for example, half-filled 2p orbital is Nitrogen and completely
filled orbitals in Neon are given as follows.
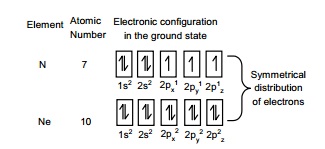
Thus the p3,p6,d5,d10,f7
and f14 configuration
which are either completely filled or exactly half-filled are more stable.
Further, it may be noted that chromium and copper have five and ten
electrons in 3d orbitals rather than
four and nine electrons respectively as expected. Therefore, to acquire more
stability one of the 4s electron goes into 3d
orbitals so that 3d orbitals get
half-filled or completely filled in chromium and copper respectively.
Chromium
Expected
configuration : 1s2,2s2,2p6,3s2,3p6,3d4,4s2
Actual configuration : 1s2,2s2,2p6,3s2,3p6,3d5,4s1
![]() Electron
exchange
Electron
exchange
Copper
Expected
configuration : 1s2,2s2,2p6,3s2,3p6,3d9,4s2
Actual configuration : 1s2,2s2,2p6,3s2,3p6,3d10,4s1
![]() Electron
exchange
Electron
exchange
Related Topics