Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Aufbau Principle
Aufbau Principle
The word 'aufbau' in German means 'building up'. The building up of
orbitals means the filling up of orbitals with electrons. The principles
states: In the ground state of the
atoms, the orbitals are filled in order of their increasing energies. In
other words, electrons first occupy
the lowest-energy orbital available to them and enter into higher energy
orbitals only after the lower energy orbitals are filled. The order in which
the energies of the orbitals increase and hence the order in which the orbitals
are filled is as follows:
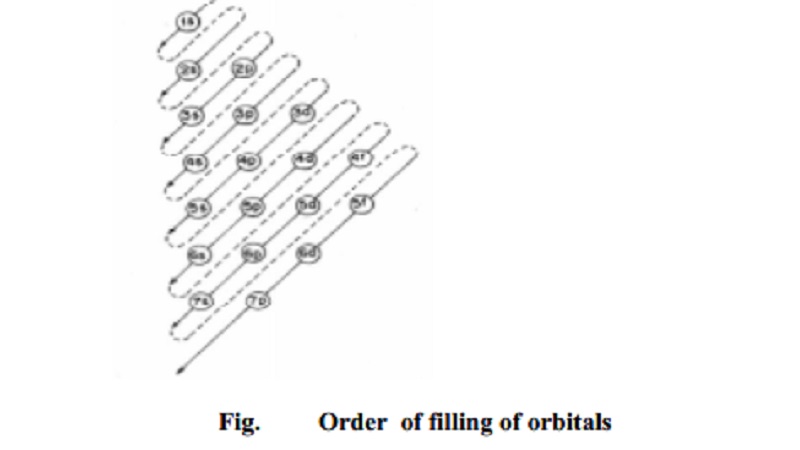
1s, 2s,
2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s…………….
This order may be remembered by using the method
given in Fig. 3.3. Starting from the top, the direction of the arrows gives the
order of filling of orbitals. Alternatively, the order of increase of energy of
orbitals can be calculated from (n +1) rule, explained below:
The lower the value of (n+1) for an orbital, the
lower is its energy. If two orbitals have the same (n+1) value, the orbital
with lower value of n has the lower energy.
It may be noted that different subshells of a particular shell have
different energies in case of many-electron atoms. However in hydrogen atom,
they have the same energy.
Related Topics