Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Hund's rule of maximum multiplicity
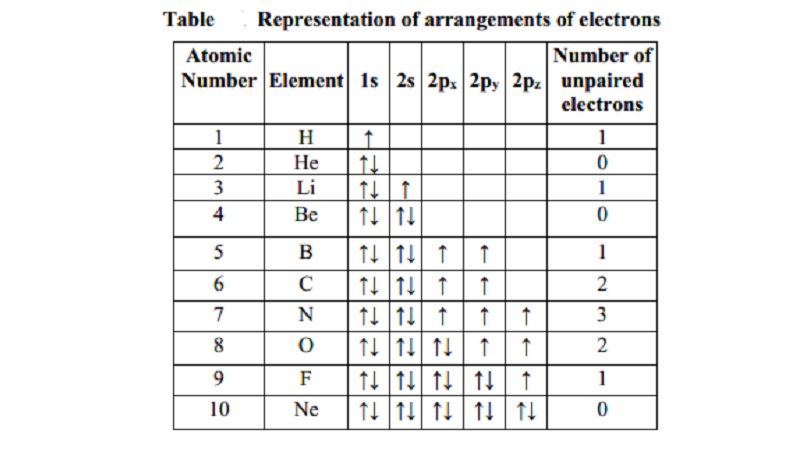
Hund's rule of maximum multiplicity
Hund's
rule of maximum multiplicity states, that in filling p, d or f orbitals, as
many unpaired electrons as possible are placed before pairing of electrons with
opposite spin is allowed. Pairing of electrons requires energy. Therefore no pairing occurs until all orbitals of a
given sub-level are half filled. This is known as Hund's rule of maximum multiplicity. It states that when
electrons enter sub-levels of fixed (n+1) values , available orbitals are
singly occupied (Table).
Atomic Number
of
Number Element 1s 2s 2px 2py 2pz Unpaired
Electrons
1 H up 1
2 He both 0
3 Li both up 1
4 Be both both 0
5 B both both up up 1
6 C both both up up 2
7 N both both up up up 3
8 O both both both up up 2
9 F both both both both up 1
10 Ne both both both both both 0
Thus, if three electrons are to be filled in the
p- level of any shell, one each will go into each of the three (px, py, pz)
orbitals. The fourth electron entering the p- level will go to px
orbital which now will have two electrons with opposite spins (as shown above)
and said to be paired. The unpaired electrons play an important part in the
formation of bonds.
Related Topics