Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Single Electrode Potential
SINGLE ELECTRODE POTENTIAL
An electrochemical cell consists of two
half-cells. With an open-circuit, the metal electrode in each half-cell
transfers its ions into solution. Thus an individual electrode develops a
potential with respect to the solution. The potential of a single electrode in
a half-cell is called the Single
electrode potential. Thus in
Daniel cell in which the electrodes are not connected externally, the anode Zn/Zn2+ develops a negative
charge and the cathode Cu/Cu2+, a positive charge. The amount of the
charge produced on an individual electrode determines its single electrode
potential.
The single electrode potential of a half-cell
depends on : (a) concentration of ions in solution ; (b) tendency to form ions
; and (c) temperature.
Standard emf of a cell
The emf generated by an electrochemical cell is
given by the symbol E. It can be measured with the help of a potentiometer. The
value of emf varies with the concentration of the reactants and products in the
cell solutions and the temperature of the cell. When the emf of a cell is determined
under standard conditions, it is called the standard emf. The standard
conditions are : (a) 1 M solutions of reactants and products ; and (b)
temperature of 25o C. Thus standard emf may be defined as the emf of
a cell with 1 M solutions of reactants and products in solution measured at 25o
C. Standard emf of a cell is represented by the symbol Eo. For gases
1 atm. pressure is a standard condition instead of concentration. For Zn-Cu
voltaic cell, the standard emf, Eo is 1.10V.
Zn | Zn2+(aq, 1M) || Cu2+(aq,
1M) | Cu Eo = 1.10 V
Determination of emf of a
half-cell
By a single electrode potential, we also mean
the emf of an isolated half-cell or its half-reaction. The emf of a cell that
is made of two half-cells can be determined by connecting them to a voltmeter.
However, there is no way of measuring the emf of a single half-cell directly.
The emf of the newly constructed cell, E is determined with a voltmeter. The
emf of the unknown half-cell Eo can then be calculated from the
expression
Emeasured = ER -
E L
If the standard half-cell acts as anode, the
equation becomes
ER = Emeasured (Q EL = 0)
On the other hand, if standard half-cell is
cathode, the equation takes the form
EL = - E measured (Q ER = 0)
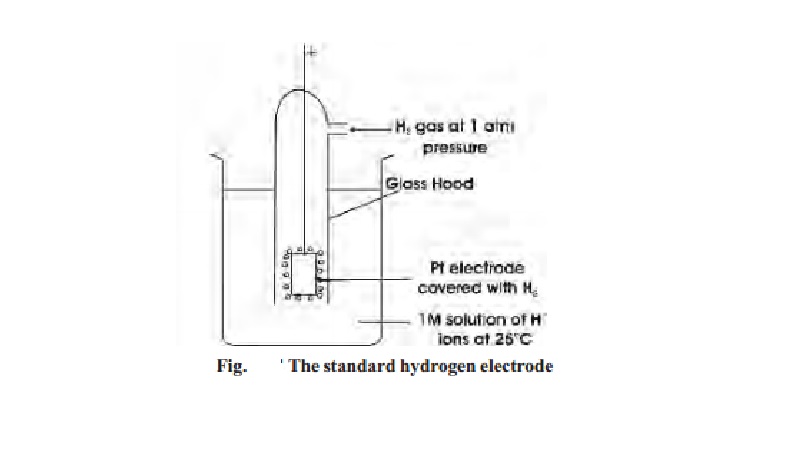
The standard hydrogen half-cell or Standard
Hydrogen Electrode (SHE), is selected for coupling with the unknown half-cell.
It consists of a platinum electrode immersed in a 1 M solution of H+
ions maintained at 25 oC. Hydrogen gas at one atmosphere enters the
glass hood and bubbles over the platinum electrode. The hydrogen gas at the
platinum electrode passes into solution, forming H+ ions and
electrons.
The emf of the standard
hydrogen electrode is arbitrarily assigned the value of zero volts. So, SHE can
be used as a standard for other electrodes.
The half-cell whose potential
is desired, is combined with the hydrogen electrode and the emf of the complete
cell determined with a voltmeter. The emf of the cell is the emf of the
half-cell.
For example, it is desired to determine the emf
of the zinc electrode, Zn | Zn2+. It is connected with the SHE. The
complete electrochemical cell may be represented as :
Zn | Zn2+ || H+ | H2 (1 atm), Pt
The emf of the cell has been found to be -0.76
V which is the emf the zinc half-cell. Similarly, the emf of the copper
electrode, Cu2+ | Cu can be determined by pairing it with the SHE
when the electrochemical cell can be represented as :
Pt,
H2 (1 atm) | H+ || Cu2+ | Cu
The emf of this cell has been determined to be
0.34 V which is the emf of the copper half-cell.
Eocell = EoCu/Cu2+
- E
=0.34 - Zero
=0.34 V
The two situations are explained as follows :
When it is placed on the right-hand side of the
zinc electrode, the hydrogen electrode reaction is
2H+ + 2e- ---- > H2
The electrons flow to the SHE and it acts as
the cathode.
When the SHE is placed on the left hand side,
the electrode reaction is
H2 -- -- -- > 2H+ + 2e-
The electrons flow to the copper electrode and
the hydrogen electrode
as the anode. Evidently, the SHE can act both
as anode and cathode and, therefore can be used to determine the emf of any
other half-cell electrode (or single electrode).
According to IUPAC
convention, the standard reduction potentials alone are the standard
potentials. The values of the standard potentials at 25oC (298 K)
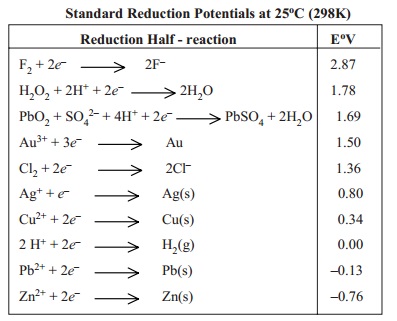
for some common Reduction Half-reactions are listed in Table below.
Standard
Reduction Potentials at 25oC (298K)
Predicting Cell EMF
The standard emf Eo,
of a cell is the standard reduction potential of right-hand electrode (cathode)
minus the standard reduction potential of the left-hand electrode (anode). That
is,
Eocell = Eoright - E oleft
= Cathode potential - Anode potential
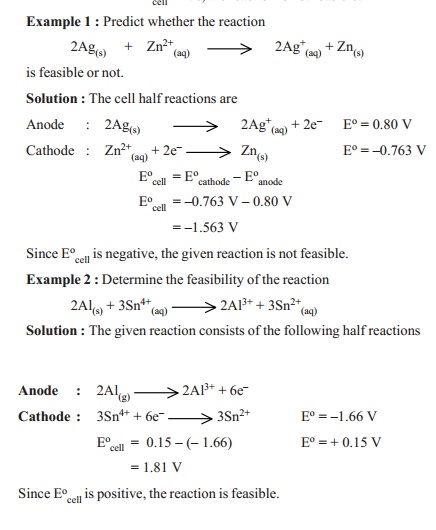
Let us predict the emf of the cell
Zn(s) | Zn2+(aq) || Ag+(aq) | Ag
by using the Eo values from the table.
Eocell = EoR - E oL
0.80 - (- 0.763)
0.80 + 0.763 = 1.563 V
Predicting Feasibility of
Reaction
The feasibility of a redox reaction can be
predicted with the help of the electrochemical series. The net emf of the cell
reaction, Ecell, can be calculated from the expression
Eo
cell = Eo cathode - Eo anode
In
general, if Eocell = + ve, the reaction is feasible
Eo
cell = -ve, the reaction is not feasible
Related Topics