Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Scope and Nature of Chemical Equilibrium
Equilibrium in
Chemical Reactions
Consider a chemical reaction between A and B to
form products C and D. After allowing sufficient period of time for the
reaction, upon analyses, when A and B are absent in the reaction mixture, then
the reaction is understood to be complete and only the presence of C and D will
be detected. For example, when sodium reacts with water, sodium hydroxide and
hydrogen gas are produced, and the reverse reaction to form back the reactants
never occurs even when the reaction vessel is a closed one. Reactions when go
to completion and never proceed in the reverse direction are called as irreversible reactions. The chemical
equations of such reactions are represented with a single arrow as A + B -- > C + D.
For Example. 2 Na + 2 H2O -- > 2 NaOH + H2
However, even after allowing sufficient period
of time for reaction, when the presence of A and B are always detected along
with C and D, then such reactions are understood to be never complete.
For example, when H2 and I2 are reacted, 2 HI is
formed. Initially the reaction proceeds to form HI until a certain period of
time and with further increase in the reaction time, HI molecules dissociate to
produce back H2 and I2 in such a way that, the reaction mixture always contain
H2, I2 and HI for any length of time until external factors like temperature,
pressure, catalyst etc. are applied. Reactions which never proceed to
completion in both forward and backward direction are called as Equilibrium reactions.
The chemical equation of such reactions are represented as,
A + B -- > < -- C + D
Example H2 + I2
-- > < --- 2 HI
when both forward and reverse reaction rates are equal, the
concentration of reactants and products do not change with any length of
reaction time. Physical transformations of matter like change of solid to
liquid states or liquid to vapour states also take place under equilibrium
conditions with both the states of matter being present together. For example,
at 0C, melting
ice and freezing water are both present.
Scope of Chemical
Equilibrium
Study of chemical equilibria possesses many
scopes. The knowledge on whether the equilibrium lies in favour of reactants or
products under certain experimental conditions is useful to increase yields in
industrial processes, to establish the exact proton transfer equilibria in
aqueous protein solutions. Since small changes in equilibrium concentration of
hydrogen ion may result in protein denaturing and cell damage etc. This study
is also useful or certain acids, bases and salts in water exist in ionic
equilibria which control their use as buffers, colour indicators etc.
Reversible and
Irreversible Reactions
A reaction which can go in the forward and backward direction
simultaneously under the same conditions, is called a reversible reaction.
Nature of Chemical
Equilibrium
The occurrence of chemical equilibrium is seen in
reversible reactions only. Chemical equilibrium may be defined as the state of
a reversible reaction when the two opposing reactions occur at the same rate
and the concentration of reactants and products do not change with time. The
true equilibrium of a reaction can be attained from both sides.
For X -- > Y reaction,
a = - d[x] ;
b = d[Y] dt dt
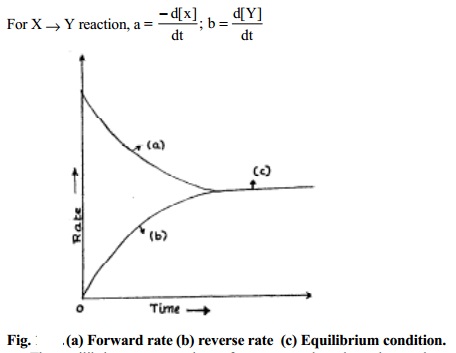
The
equilibrium concentrations of reactants and products do not change
with time. This is because, since the forward reaction rate equals with
backward reaction rate as and when the products are formed, they react back to
form the reactants in equal capacity. The equilibrium concentrations of
reactants are different from their initial concentrations.
The equilibrium concentrations are represented
by square brackets with subscript `eq' or as [ ]eq. Thus [A]eq denotes the
equilibrium concentration of A in moles per litre. In modern practice, the
subscript `eq' is not used.
Related Topics