Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Dynamic Equilibrium: Characteristics, Equilibrium in physical and chemical processes
Dynamic Equilibrium
When a reversible reaction attains equilibrium
it appears that the concentrations of individual reactants and that of the
products remain constant with time. Apparently, the equilibrium appears as dead
(or) as not proceeding. Actually, the reactant molecules are always reacting to
form the product molecules. When the product molecules are able to react with
themselves under the same experimental condition to form the same amount of
reactants simultaneously (at the same time) in an equal rate of the forward reaction,
then the process is a ceaseless phenomenon. Thus chemical equilibrium is dynamic when the forward and reverse
reactions take place endlessly and
simultaneously with equal rates. Therefore chemical equilibrium is called
as dynamic equilibrium.
Characteristics of
Chemical Equilibrium
(i) Constancy of concentrations
When a chemical equilibrium is established in a closed vessel at
constant temperature, the concentrations of various species like reactants and
products remain unchanged.
The reaction mixture consisting of reactants and products at equilibrium
is called as equilibrium mixture.
The concentrations of reactants and products at equilibrium are called
as equilibrium concentrations.
(ii) Equilibrium can be initiated from either
side. The state of
equilibrium of a reversible reaction can be arrived at whether we start
from reactants or products.
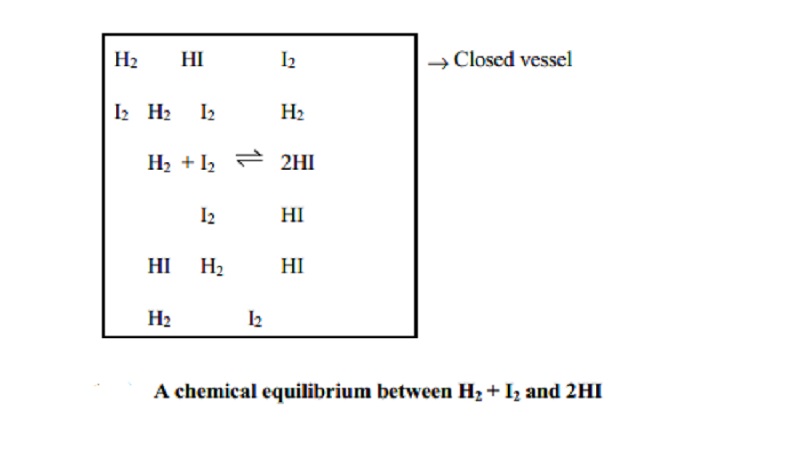
For example, this equilibrium H2(g) + I2(g) _ 2HI(g) can be achieved
whether we start with H2 and I2 or with HI.
(iii) Equilibrium cannot be attained in an open vessel
Only in a closed vessel, a reaction can be
considered to attain equilibrium since no part of reactants or products should
escape out. In an open vessel, gaseous reactants or products may escape so that
no possibility of attaining equilibrium exists. Equilibrium can be attained
when all the reactants and products are in contact with each other.
(iv) Catalyst does not alter the equilibrium
When a catalyst is added to the equilibrium
system, it speeds up the rates of both forward and reverse reactions to an
equal extent. Therefore the equilibrium is not changed but the state of
equilibrium is attained earlier.
(v) The
value of equilibrium constant does not depend upon the initial concentration of
reactants.
(vi) At
equilibrium, the free energy change is minimum or zero.
(vii)When temperature is changed, the forward and backward reaction
rates are changed and the equilibrium concentrations of reactants and products
are changed.
Equilibrium in
physical processes
When there
is a change in the state of occurrence of matter, then a physical
transformation is said to have occurred. The equilibrium concepts are also
applicable to physical state transformations of matter.
(i)
Solid-liquid equilibria
Here, the
solid and the liquid forms of a substance co exist at characteristic
temperature and pressure. At 1 atm and at the melting point of a substance,
there is a solid-liquid equilibrium existing. For example, the solid-liquid
equilibrium of water at 0OC,
water(l) -- > < --- ice(s)
occurs at
1 atm pressure. Here, both the liquid and ice exist together. Also, at melting
point of ice or freezing point of water, the rate of melting of ice equals with
rate of freezing of water. With change in pressure the temperature at which
this equilibrium onsets changes.
(ii) Liquid-vapour equilibrium
Here the
vapour and the liquid forms of a substance exist simultaneously at a
characteristic temperature called as boiling point and at 1 atm pressure. For
example at 100oC which is
the boiling point of water, and 1 atm pressure,
Water(l) -- > < -- Steam(g)
both
liquid water and water vapour (steam) exist simultaneously, provided the vapour
does not escape.
(iii)Solid-solid equilibrium
When a
substance existing in a particular crystalline solid transforms to another
crystalline form retaining its solid nature at a characteristic temperature
called the transition temperature with both the solid forms coexisting, at 1
atm pressure then it is said to be in solid-solid equilibrium. For example,
solid sulphur exhibits equilibrium with rhombic to monoclinic forms at its
transition temperature.
S(rhombic) --- > < ---- S(monoclinic)
Equilibrium in chemical processes
Chemical equilibrium exists in two types such as homogeneous and
heterogeneous equilibria. In a chemical reaction existing in equilibrium, if
all the reactants and products are present in the same phase, then a
homogeneous equilibria is said to have occurred.
For example,
N2(g) + 3H2(g) -- > < -- 2NH3(g).
Here all the reactants and products exist in gaseous state. This is an
example of gas-phase equilibrium.
The chemical equilibrium in which all the reactants and products are in
the liquid phase are referred to as liquid equilibria. For example,
CH3 COOH(l) + C2 H5 OH(l) CH3 COOC2 H5(l) + H2O(l)
Both gas phase and liquid phase equilibria are collectively called as
homogeneous equilibria.
Heterogeneous equilibrium
In a chemical equilibrium, if the reactants and products are in
different phases then heterogeneous equilibrium is said to have occurred.
Examples :
CaCO3(s) -- > < -- CaO(s)
+ CO2(g)
3Fe(s) + 4H2O(g) -- > < -- Fe3O4(s)
+ 4H2(g)
Here, only when the reaction is carried out in closed vessel, the
equilibrium state is established.
Related Topics