Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Raoult's Law and Dynamic method (or) Ostwald - Walker method
Raoult's Law
The relationship between the vapour pressure of the solution and its
concentration is given by a French chemist named Francois Marie Raoult (1886).
According to Raoult's law, at constant temperature the vapour pressure of the
solution (p) is directly proportional to the molefraction of the solvent (X1)
present in the solution. That is, p . ;1 (or) p = kX1 where k is the proportionality constant. The value of k
is known as follows: For a pure solvent, X1 = 1.0 and p becomes p o corresponding to the vapour pressure of the
pure solvent. Thus, p o = k
(1.0). Substituting the value of k,
p = p o X1
------- 1
Equation 1 is generally known as Raoult's law.
When n1 and n2 are the number of moles of
solvent and solute present in the solution, the molefraction of the solvent X1
= n1/(n1 + n2) and the mole fraction of solute X2 = n2/(n1+n2). Also, X1 + X2 =
1.0
If W1 and W2 are the weights of solvent and
solute present, then n1 = W1/M1 and n2 = W2/M2. M1 and M2 are the molar masses
of solvent and solute respectively.
It is generally observed that p is lower than P o . The lowering of vapour pressure of the solvent
in the solution equals to (p o - S ûS
The relative lowering of the vapour pressure, is
defined as the ratio of the lowering of vapour pressure to the vapour pressure
of the pure solvent. Thus relative lowering of vapour pressure is given by
p o -p /P o = ∆P / P o
p o -p / p o
= p o -p o X1 / p o since
p = p o X1
P o (1-X1) / P o = 1-X1
= X2.... since X1+X2 = 1
{ }
p o -p /
p o = X2 ------- 2
Equation 2 represents the mathematical from of Raoults law. Thus, the
statement of Raoult's law for dilute solutions containing non-volatile
non-electrolyte solute is: Relative lowering of vapour pressure is equal to the
mole fraction of the solute. Since mole fraction of the solute (X2)
is given by n2/(n1+n2), the quantity (p-p)/p depends upon the number of moles or molecules of the solute in solution
and not on its chemical nature. Thus, relative lowering of vapour pressure is a
colligative property.
Determination of molecular weights from relative lowering of vapour
pressure
In dilute solutions, the number of moles of solvent (n1) is large
compared to the number of moles of solute (n2) and thus (n1
+ n2) can be approximated to n1 and x2 becomes
equal to n2/n1.
Thus ∆p /p0 = n2
/ n1 = W2.M1
/ M2.W1
---------3
Substituting for n1 and n2 as
W1/M1 and W2/M2 we get
∆p /p0 = M1.W2/W1.M2. Knowing M1,W1 and
W2 and from the measurement of lowering of vapour pressure, M2 the molar
mass of the solute can be determined using equation.3.
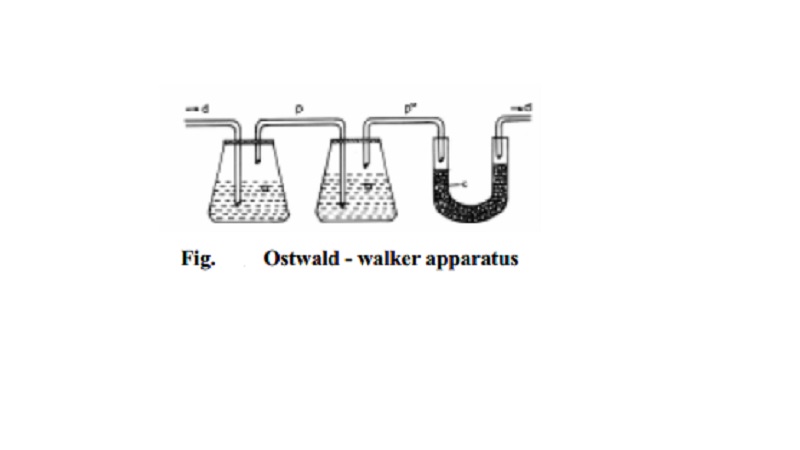
Dynamic method (or)
Ostwald - Walker method
This method is based on the principle that when dry air is successively
passed through a series of containers possessing solution and pure solvent
respectively, the air becomes saturated with the solvent vapours and an equal
amount of weight loss in solution and solvent containers takes place.
In Fig. the first chamber (a) contains a weighed amount of the solution
under examination and the next chamber (b) contains a weighed amount of the
pure solvent. A weighed amount of anhydrous and dry calcium chloride is taken
in the U-tube (c) connected at the end. The chambers and the U-tube are
connected by a series of delivery tubes
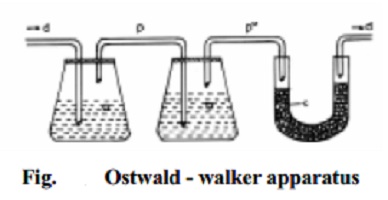
(d) through which air is passed. The dry air is first allowed to pass
through the solution chamber until the air is saturated with the solvent vapour
to maintain the vapour pressure of the solution `p'. Consequently, a loss in
weight of the solution results in the solution chamber since some amount
solvent molecules have evaporated. When this air is allowed to pass through the
pure solvent chamber some more solvent vapour gets in stream with air, until
the vapour pressure of pure solvent p o , is maintained. This happens so because p o is greater than p. Consequently, the weight loss registered in the
solvent chamber is proportional to the (p o -p) quantity.
The weight loss in solution chamber . S
The weight loss in solvent chamber . S o -p
Sum of the
loss in weights of solution
and solvent chamber . (p+p o -p) . S o
When the air saturated with solvent vapours is passed through CaCl2
U-tube, the solvent vapours are absorbed and the dry air gets out. The gain in
weight of the CaCl2 U-tube should be equal to the total loss in
weight of solution and solvent chambers, which is inturn proportional to p o .
Loss in weight of the solvent chamber / Gain in weight of CaCl2
tube = p o -p / p o
= relative lowering of the vapour pressure
Thus, using the experimental (p o -p)/p o values
and applying Raoult's law, the molecular weight of the solute can be
determined.
Related Topics