Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Depression of freezing point of dilute solution
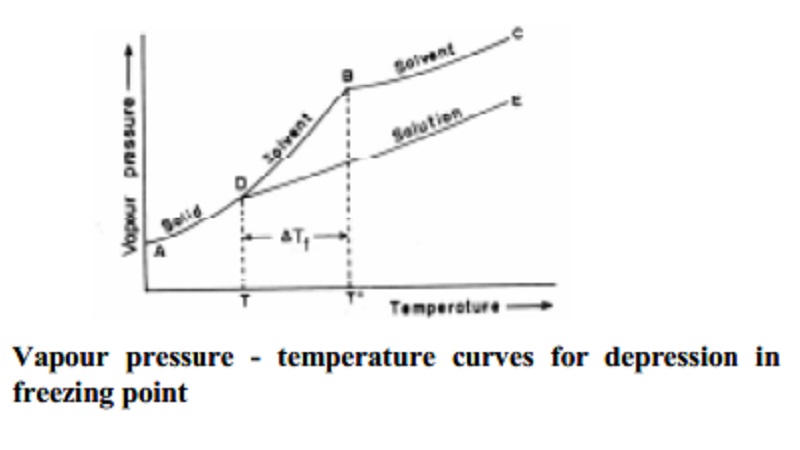
Depression of freezing point of dilute solution
Freezing
point is the temperature at which solid and liquid states of a substance have
the same vapour pressure. According to Raoult's law, addition of a non-volatile
solute to solvent lowers the vapour pressure of the solvent and hence, the
vapour pressure of pure solvent is greater than the vapour pressure of
solution. Thus the temperature at which the solution and its solid form
existing in equilibrium and possessing the equal vapour pressures, is lowered.
That is, the freezing point of solution is lowered. The lowering of the
freezing point of the solution from that of the freezing point of the pure
solvent is known as depression in freezing point of the solution.
Consider the vapour pressure curves shown in Fig.. Generally when the
temperature of a solid substance that is used as the solvent is raised, the
vapour pressure also raises. AB curve depicts this. Similarly curve BC
represents the increase in vapour pressure of the liquid solvent with increase
in temperature. Curves AB and BC meet at B corresponding to To
temperature which is the freezing point of the pure solvent. At To,
the vapour pressure of the liquid and solid states of the solvent are equal at
B. Since the vapour pressure of the solution is always lower than that of its
pure solvent, the vapour pressure curve of the solution DE always lie below
that of the pure solvent.
D is the
point of intersection of the vapour pressure curves of solution and pure
solvent. The temperature at D is the freezing point of the solution and is seen
to be lower than To. The depression in freezing point is ∆Tf=T0-T.
The measured depression in freezing point (∆Tf) is found to be directly
proportional to the molality (m) of the solute in solution. That is, ∆Tf props to m or ∆Tf - Kfm
, where Kf is called s the the cryoscopic constant (or)
molal freezing point depression constant. `Kf'
is defined as the depression in
freezing point produced when one mole of solute is dissolved in 1 kg solvent.
It is also the depression in freezing point of one molal solution. Freezing
point depression of a dilute solution is found to be directly proportional to
the number of moles (and hence the no.of molecules) of the solute dissolved in
a given amount of the solvent. Also ∆Tf is independent of the nature of the solute as
long as it is non-volatile. Hence depression in freezing point is considered as a colligative
property.
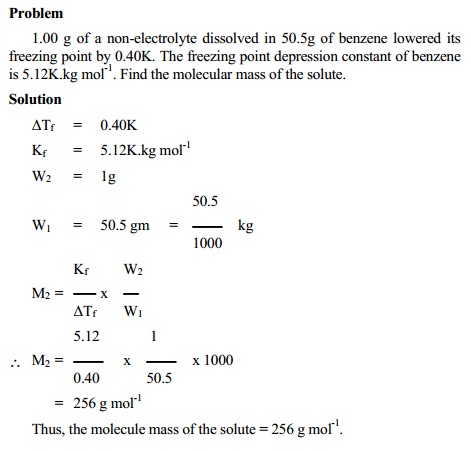
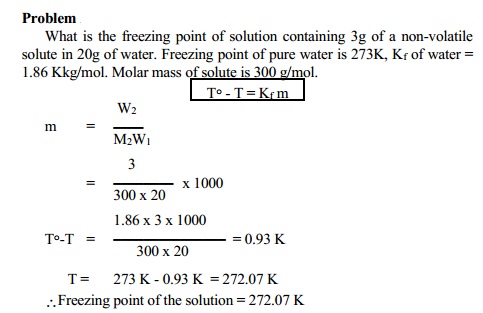
Determination of molecular weight from
depression in freezing point
∆Tf = Kf.m
Where m = molality
M = n2 / W1 nad n2 = W2 / M2
W1 = Weight of the solvent in Kg;
M2 = Molecular weight of solute
m = W2 / M2W1
∆Tf = Kf. (W2 / M2W1)
M2 = Kf W2 / ∆Tf W1 - K.kg mol-1g/ K.kg
M2 = Kf W2 / ∆Tf W1 - g
mol-1
Thus the molecular weight of the solute can be calculated.
Related Topics