Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Measurement of freezing point depression by Beckmann method
Beckmann Method
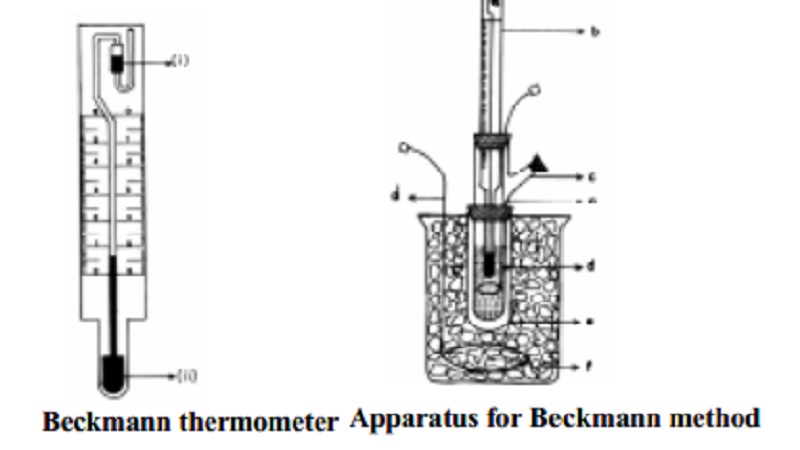
Beckmann thermometer is used to measure small temperature changes in the
freezing point of pure solvent and solution. Beckmann thermometer is not used
in determining the absolute value of freezing temperature of the solvent or
that of the solution. It is therefore called a differential thermometer.
Temperature differences of even 0.01K can easily be measured.
Beckmann thermometer (Fig.11.4) consists of a large thermometer bulb at
the bottom of a free capillary tube (ii) which is connected to a reservoir of
mercury (i) placed at the top. As the capillary has fine bore, a small change
of temperature causes a considerable change in the height of mercury column
(level) in the capillary. The whole scale of a Beckmann thermometer covers only
about 6K. Initially the level of mercury in the capillary should be on the
scale. This is achieved by transferring mercury from the lower bulb to the
reservoir and viceversa. When the Beckmann thermometer is used at high
temperatures, some of the mercury from the thermometer bulb is transferred into
the upper reservoir. At lower temperature mercury from the reservoir falls down
in to the thermometer bulb.
Measurement of freezing point depression by
Beckmann method
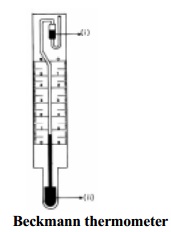
A simple Beckmann apparatus is shown in Fig.11.5. It consists of a
freezing tube (a) with a side arm (c) through which a known amount of a solute
can be introduced. A stopper carrying a Beckmann thermometer (b) and a stirrer
(d) is fitted in to the freezing tube. To prevent rapid cooling of the contents
of the freezing tube, A, a guard tube (e) surrounds the tube so that there is
an air space between a and e. This assembly, as a whole, is placed in a wide
vessel V which contains a freezing mixture (f) maintaining a low temperature
around 5C below
the freezing point of the pure solvent.
A known weight of the pure solvent is placed in the tube (a). It is
cooled with gentle and continuous stirring. As a result of super cooling, the
temperature of the solvent will fall by about 0.5 deg C below its freezing point. Vigorous stirring is then set in when solid
starts separating and the temperature rises to the exact freezing point. This
temperature remains constant, for some time, until all the liquid solvent gets
solidified and is noted as To.
The tube (a) is taken out, warmed to melt the solid and a known weight
of the solute is added through the side arm (c). When the solute is dissolved
in to the solvent forming a solution, the tube (a) is put back in to the original
position and the freezing point of the solution (T) is redetermined in the same
manner as before. The difference between the two readings gives the freezing
point depression (∆Tf).
Depression in freezing point ∆Tf = To-T.
From this value, the molecular mass of the non-volatile solute can be determined using the expression
and known Kf value.
M2 = Kf . W2
/ ∆Tf .W1
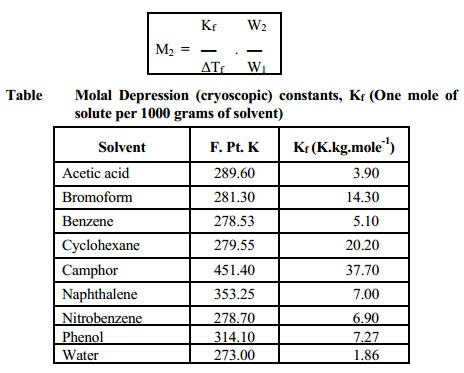
Solvent F. Pt. K Kf (K.kg.mole-1)
Acetic acid 289.60 3.90
Bromoform 281.30 14.30
Benzene 278.53 5.10
Cyclohexane 279.55 20.20
Camphor 451.40 37.70
Naphthalene 353.25 7.00
Nitrobenzene 278.70 6.90
Phenol 314.10 7.27
Water 273.00 1.86
Related Topics