Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Lowering of Vapour Pressure
Lowering
of Vapour Pressure
If we take a pure liquid in a closed container,
we find that a part of the liquid evaporates and fills the available space with
its vapour. The vapour exerts a pressure on the walls of the container and
exists in equilibrium with the liquid. This pressure is referred as the vapour
pressure of the liquid.
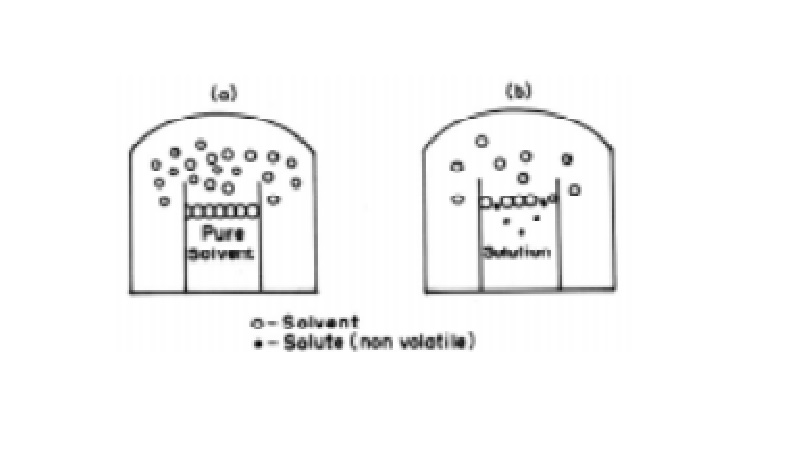
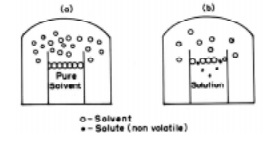
When a non-volatile solute is dissolved in the
solvent so that a dilute and homogeneous solution results, then again the
vapour pressure of the solution will be made up of entirely from the solvent
since the solute does not evaporate. This vapour pressure of the dilute
solution is found to be lower than the vapour pressure of the pure solvent.
From Fig.
it may be seen the surface of a dilute solution is partly occupied by solute
molecules, thereby the number of solvent molecules at the surface being
reduced. Consequently the vapour pressure of the solvent molecules gets lowered
on the surface of the solution.
Raoult's Law
Related Topics