Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Purification Methods Of Sols
PURIFICATION OF SOLS
In the methods of preparation stated above, the resulting sol frequently
contains besides colloidal particles appreciable amounts of electrolytes. To
obtain the pure sol, these electrolytes have to be removed. This purification
of sols can be accomplished by three methods:
i.
Dialysis
ii.
Electrodialysis and
iii.
Ultrafiltration
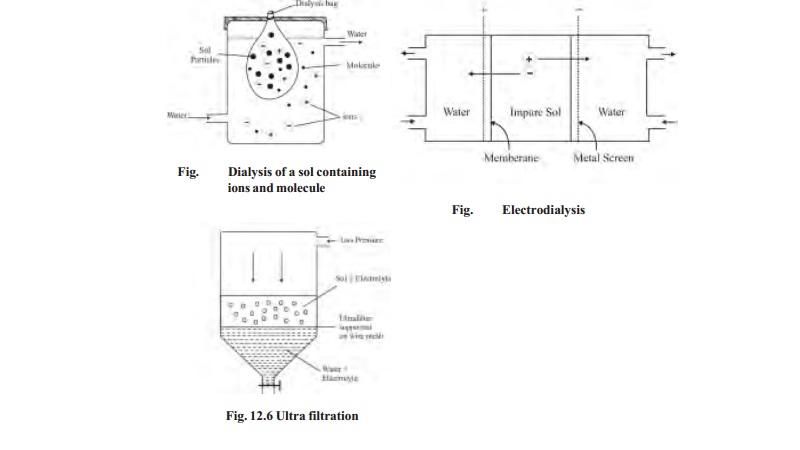
i) Dialyser
Animal
membranes (bladder) or those made of parchment paper and cellophane sheet, have
very fine pores. These pores permit ions (or small molecules) to pass through
but not the large colloidal particles.
When a sol containing dissolved ions
(electrolyte) or molecules is placed in a bag of semipermeable membrane dipping
in pure water, the ions diffuse through the membrane. By using a continuous
flow of fresh water, the concentration of the electrolyte outside the membrane
tends to be zero. Thus diffusion of the ions into pure water remains brisk all
the time. In this way, practically all the electrolyte present in the sol can
be removed easily. The process of removing ions (or molecules) from a sol by
diffusion through a permeable membrane is called Dialysis. The apparatus used for dialysis is called a Dialyser.
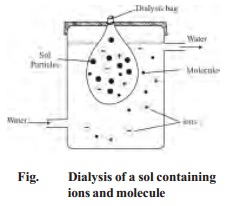
ii) Electrodialysis
In this
process, dialysis is carried under the influence of electric field. Potential
is applied between the metal screens supporting the membranes. This speeds up
the migration of ions to the opposite electrode. Hence dialysis is greatly
accelerated. Evidently electrodialysis is not meant for non-electrolyte
impurities like sugar and urea.
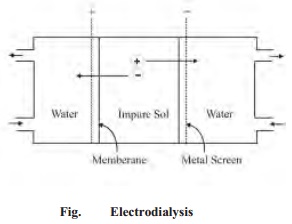
iii) Ultrafiltration
Sols pass
through an ordinary filter paper. Its pores are too large to retain the
colloidal particles. However, if the filter paper is impregnated with collodion
or a regenerated cellulose such as cellophane or visking, the pore size is much
reduced. Such a modified filter paper is called an ultrafilter.
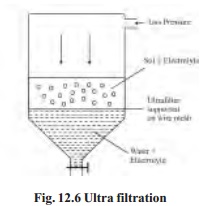
The
separation of the sol particles from the liquid medium and electrolytes by
filtration through an ultrafilter is called ultrafiltration.
Ultrafiltration
is a slow process. Gas pressure (or suction) has to be applied to speed it up.
The colloidal particles are left on the ultrafilter in the form of slime. The
slime may be stirred into fresh medium to get back the pure sol. By using
graded ultrafilters, the technique of ultrafiltration can be employed to
separate sol particles of different sizes.
Related Topics