Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Preparation Of Colloids : Dispersion and Condensation method
PREPARATION
OF COLLOIDS
1.Preparation
of lyophilic sols: The
colloidal solutions of lyophilic colloids
like starch, glue, gelatin etc., can be readily prepared by dissolving these
substances in water either in cold or on warming.
2.Preparation
of lyophobic sols : Lyophobic
sols are prepared by special methods.
These methods fall into two categories.
i.
Dispersion
methods: By splitting coarse aggregates of a substance into a colloidal size.
ii.
Condensation
methods: By aggregating very small particles into the colloidal particles.
I. Dispersion method
1. Mechanical dispersion
2. Electro-dispersion
3. Ultrasonic dispersion
4. Peptization
II.Condensation methods
1. Exchange of solvents
2. Change of physical state
3. Chemical methods
3.i. Double decomposition
3.ii. Oxidation
3.iii. Reduction
3.iv. Hydrolysis
I. Dispersion
Methods
1.
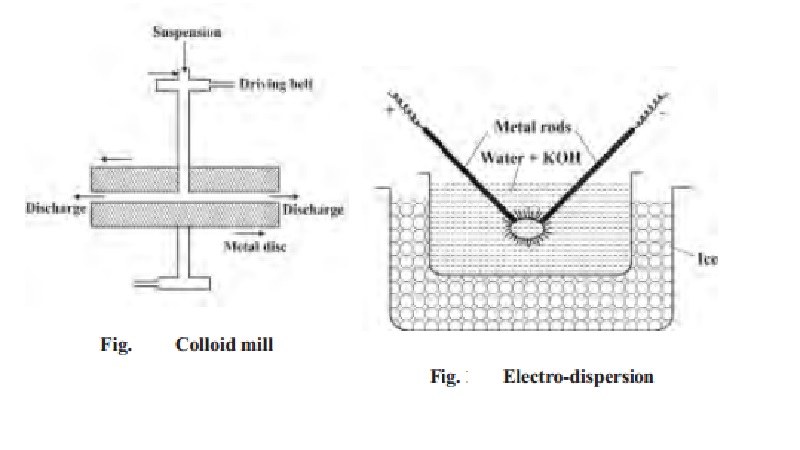
Mechanical dispersion using colloidal mill
The solid along with the liquid is fed into a
colloidal mill. The colloidal mill consists of two steel plates nearly touching
each other and rotating in opposite directions with high speed. The solid
particles are ground down to colloidal size and then dispersed in the liquid.
Colloidal graphite and printing inks are made by this method.
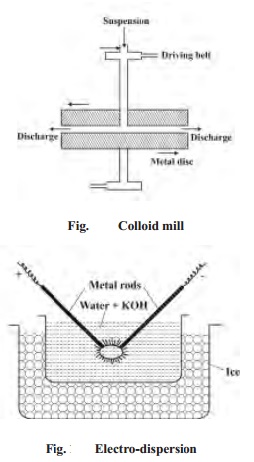
2. Electro-dispersion
method: (Bredig's Arc Method)
This method is suitable for the preparation of
colloidal solution of metals like gold, silver, platinum etc. An arc is struck
between the metal electrodes under the surface of water containing some
stabilising agent such as trace of alkali. The water is cooled by immersing the
container in a cold bath. The intense heat of the arc vapourises some of the
metal which condenses under cold water.
3.
Ultra-sonic dispersion :
The sound waves of high frequency are usually
called ultra-sonic waves. Ultrasonic waves are passed through the solution
containing larger particles. They break down to form colloidal solution.
4.
Peptisation :
The dispersion of a precipitated material into
colloidal solution by the action of an electrolyte in solution is termed as
peptisation. The electrolyte used is called a peptizing agent.
A few examples are
Silver
chloride can be converted into a sol by adding hydrochloric acid
Ferric
hydroxide yields a sol by adding ferric chloride
II. Condensation
methods
1. By
exchange of solvent
If a solution of sulphur or phosphorus in
alcohol is poured into water, a colloidal solution of sulphur or phosphorus is
obtained due to low solubility in water.
2. By
change of physical state
Colloidal solutions of certain elements such as
mercury and sulphur are obtained by passing their vapour through cold water
containing a stabiliser.
3.
Chemical Methods
The
chemical methods involve chemical reaction in a medium in which the dispersed
phase is sparingly soluble. Some of the methods are
(i) Double decomposition: An Arsenic
sulphide sol is prepared by passing a slow stream of hydrogen sulphide gas
through a cold solution of arsenious oxide. This is continued till the yellow
colour of the sol attains maximum intensity.
As2O3 + 3 H2S --- --- -- > As2S3(yellow) + 3 H2O
Excess hydrogen sulphide is removed by passing
in a stream of hydrogen
(ii) Oxidation : A colloidal solution of
sulphur is obtained by passing H2S into a solution of sulphur
dioxide.
2H2S
+ SO2 ---- -- - > 2H2O + 3
S
(iii) Reduction: Silver sols and gold sols can
be obtained by treating dilute solution of silver nitrate or gold chloride with
organic reducing agents like tannic acid or formaldehyde.
AgNO3
+ tannic acid --- -- > Ag Sol
AuNO3
+ tannic acid --- -- > Au Sol
(iv) Hydrolysis: Colloidal solutions of the hydroxides
of Fe, Cr, Al etc can be prepared by hydrolysis of their salts. A colloidal
solution of ferric hydroxide is obtained by boiling a dilute solution of ferric
chloride.
FeCl3
+ 3H2O -- --- - -> Fe(OH)3(red
sol) + 3HCl
Related Topics