Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Colloids and Types of Colloids
COLLOIDS
Thomas Graham, in 1861, during his work on diffusion, found that while
certain substances, such as sugars, salts, acids and bases diffused readily
through a parchment membrane, others, such as gelatin, albumen and glue,
diffused at a very slow rate. The substances belonging to the former category which
generally exist in crystalline state, were called crystalloids while the
substances belonging to the second category were given the name colloids. These
observations led to the development of a new branch known as colloidal science.
In a true solution as sugar or salt in water,
the solute particles are dispersed in the solvent as single molecules or ions.
Thus the diameter of the dispersed particles ranges from 1A o to 10A o . On the
other hand, in a suspension as sand stirred into water, the dispersed particles
are aggregates of millions of molecules. The diameter of these particles is of
the order of 2000A o or more. The colloidal solutions are intermediate between
true solutions and suspensions. When the diameter of the particles of a
substance dispersed in a solvent ranges from about 10A o to 2000A o , the system
is termed a colloidal solution.
Types of Colloids
A colloidal system is made up of two phases. The substance distributed
as the colloidal particles is called the dispersed
phase. The second continuous phase in which the colloidal particles are
dispersed is called the dispersion medium. Depending upon the physical
state of dispersed phase and dispersion medium,
the following types of colloidal solutions are possible.
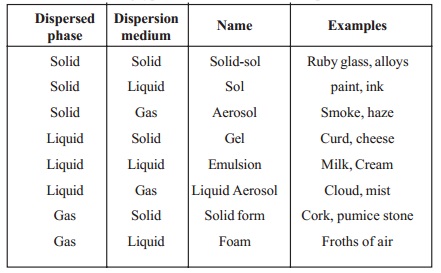
Dispersed phase + Dispersion medium = Name with Examples
Solid + Solid = Solid-sol Eg. Ruby glass, alloys
Solid + Liquid = Sol Eg. paint, ink
Solid + Gas = Aerosol
Eg. Smoke, haze
Liquid + Solid = Gel
Eg. Curd, cheese
Liquid + Liquid = Emulsion Eg. Milk, Cream
Liquid + Gas = Liquid Aerosol Eg. Cloud, mist
Gas + Solid = Solid form
Eg. Cork, pumice stone
Gas + Liquid = Foam
Eg. Froths of air
A colloidal solution of gas in gas is not
possible as gases are completely miscible and always form true solutions.
Lyophobic and
Lyophilic Colloids
Colloidal solutions in which the dispersed phase
has very little affinity for the dispersion medium are termed as lyophobic
(solvent hating) colloids. Colloidal solutions of metals which have negligible
affinity for solvents and sulphur in water are examples of this type.
Colloidal solutions in which the dispersed phase has considerable
affinity for the dispersion medium are called lyophilic (solvent loving)
colloids. Gelatin, protein and starch are examples of this type.
Related Topics