Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Theories Of Catalysis
THEORIES
OF CATALYSIS
There are two main theories to explain
catalysis.
1.
Intermediate
compound formation theory
2.
Adsorption
theory
In general, the intermediate compound formation
theory applies to homogeneous catalytic reactions and the adsorption theory
applies to heterogeneous catalytic reactions.
1. The
Intermediate Compound Formation Theory
According to this theory, the catalyst first
forms an intermediate compound with one of the reactants. The compound is
formed with less energy consumption than needed for the actual reaction. The
intermediate compound being unstable combines with other reactant to form the
desired product and the catalyst is regenerated.
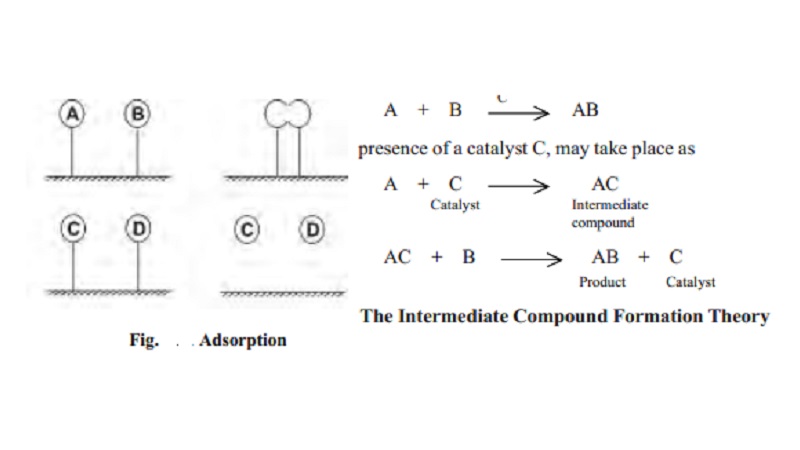
For example, a reaction of the type
A + B ---- c---- > AB
which occurs in presence of a catalyst C, may
take place as
A+C (Catalyst) ---- --- > AC (Intermediate)
AC + B --
--- --- > AB(Product) + C(Catalyst)
Many
catalytic reactions can be explained on the basis of this theory.
The catalytic oxidation of SO2 to SO3
in the lead chamber process probably takes place as;
2 NO (Catalyst) + O2 --- ---- > 2NO2 Intermediate Compound
NO2 + SO2 --- --- > SO3 (Product) +
NO (Catalyst)
2.Adsorption
Theory
This theory explains the mechanism of
heterogeneous catalysis. Here, the catalyst functions by adsorption of the
reacting molecules on its surface.
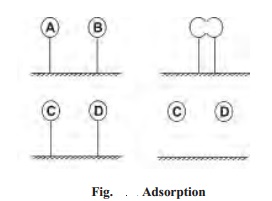
In general, there are four steps involved in the
heterogeneous catalysis.
A(g) + B(g) ----(
Catalyst) -- > C(g) + D(g)
Step
- 1. Adsorption of reactant molecules
The
reactant molecules A and B strike the surface of the catalyst. They are held up
at the surface by weak vanderwaal's forces or by partial chemical bonds.
Step
- 2. Formation of Activated complex
The particles of the reactants adjacent to one
another join to form an intermediate complex (A-B). The activated complex is
unstable.
Step
- 3. Decomposition of Activated complex
The activated complex breaks to form the
products C and D. The separated particles of the products hold to the catalyst
surface by partial chemical bonds.
Step
- 4. Desorption of Products
The particles of the products are desorbed or
released from the surface.
Applications
of catalysis
The applications of catalysis are summarised as
follows.
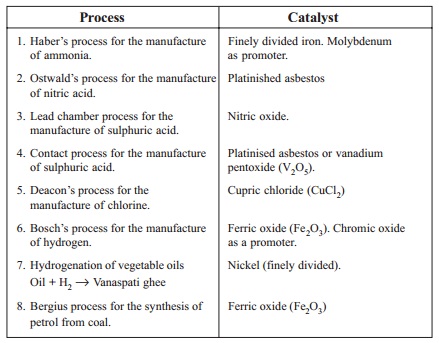
Process
i.
Haber's process for the manufacture of
ammonia.
ii.
Ostwald's process for the manufacture of
nitric acid.
iii.
Lead chamber process for the manufacture
of sulphuric acid.
iv.
Contact process for the manufacture of
sulphuric acid.
v.
Deacon's process for the manufacture of
chlorine.
vi.
Bosch's process for the manufacture of
hydrogen.
vii.
Hydrogenation of vegetable oils Oil + H2
-- > Vanaspati ghee
viii.
Bergius process for the synthesis of
petrol from coal.
Catalyst
i.
Finely divided iron. Molybdenum as
promoter.
ii.
Platinished asbestos
iii.
Nitric oxide.
iv.
Platinised asbestos or vanadium
pentoxide (V2O5).
v.
Cupric chloride (CuCl2)
vi.
Ferric oxide (Fe 2O3). Chromic oxide as
a promoter.
vii.
Nickel (finely divided).
viii.
Ferric oxide (Fe2O3)
Related Topics